Articles
MEMS-based H2 sensors improve measurement accuracy and safety
Hydrogen, more specifically green H2, is being used increasingly across the world for a wide range of industrial applications. In the U.S., forecasters predict that H2 from low-carbon sources could supply roughly 14% of the country’s energy needs by 2050, including hard-to-electrify sectors now dependent on natural gas, such as high-heat industrial processes and manufacturing fertilizer.
However, H2 is a potentially dangerous gas. An H2 leak in a refinery can rapidly escalate to a major disaster; and even in small amounts, exposure to H2 poses several different hazards to those working with it.
For decades, catalytic bead sensors and non-dispersive infrared (NDIR) sensors have been staples in the gas detection industry and have increased the safety of dangerous working environments. However, neither of these technologies are effective for use in environments where H2 is present. If pellistor/catalytic bead sensors are calibrated to H2, then detection readings are not accurate, delivering a reading of +/–30% of actual concentration. NDIR sensors cannot detect H2 because it does not absorb infrared light.
The evolution of gas sensing technology. Until recently, the gas detection industry has been relatively stagnant for nearly four decades. It was dominated by the use of Pellistor/catalytic bead and NDIR sensors. Pellistor/catalytic bead sensors were introduced nearly a century ago and still operate using the same principles found in their initial design. They measure the temperature difference between two beads—one inert and one coated in a chemical catalyst. The bead coated with the catalyst will heat more than the inert bead in the presence of many flammable gases.
Pellistor/catalytic bead sensors are appealing because they respond to a full range of flammable gases, including H2, methane, butane, propane, and carbon monoxide (CO). They are also relatively low-cost. Unfortunately, however, these sensors have critical limitations: when calibrated to a single gas, they provide inaccurate readings for all other gases; they must be calibrated frequently to perform properly; they consume relatively high power; and they do not function in low-O2 environments. However, their most significant deficiency is that they tend to become “poisoned” when exposed to high concentrations of flammable and combustible gases, or when exposed to even low concentrations of common chemicals like silicones present in many cosmetics and car waxes. When this occurs, the sensors do not work correctly, or at all, even though they may appear to be functioning normally. This scenario can put the user in a hazardous situation where they are unaware of dangerous or toxic gases present in the environment.
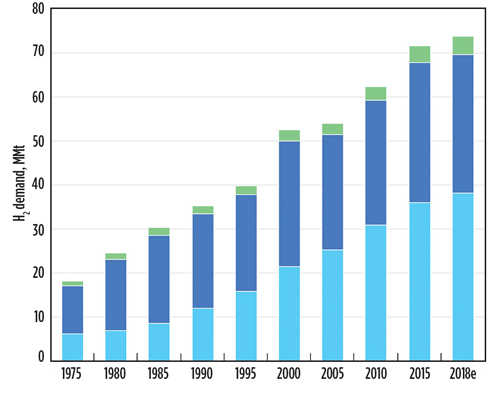
Fig. 1. Global demand for pure hydrogen, MMt, 1975–2018. Source: IEA.1
NDIR sensors were introduced and became popular for use in gas detection in the 1960s. NDIR sensors use infrared light, which is absorbed at certain wavelengths by hazardous gases. By measuring the intensity of the light transmitted through a sensing chamber containing gas and comparing it to a reference, the instrument can determine the concentration of hazardous gas present.
NDIR technology represented a huge leap in gas detection technology 40 yr ago. Today, like pellistor/catalytic bead sensors, NDIR sensors use dated technology that has serious limitations. The most significant is that they cannot detect H2, since it does not absorb infrared light.
H2 is commonly found in the mining and petroleum industries, and can be incredibly dangerous. Allowing it to go undetected may create hazardous working conditions. NDIR sensors can be calibrated to a single gas, making them ineffective at detecting gas mixtures.
NDIR sensors are also very sensitive to environmental changes. Moderate fluctuations in temperature or humidity can freeze the output reading, leaving the user unable to determine gas presence or concentration. Also, humidity, fog and ambient light can cause interference with detection if they are able to permeate the open chamber.
Additionally, while they need calibration less frequently than the pellistor/catalytic bead sensors, NDIR sensors still require calibration at least yearly. These sensors do perform better than pellistor/catalytic bead sensors in certain situations, but since NDIR is a proprietary technology, it can be expensive.
Detecting hydrogen and other hazardous gases. Detecting hazardous gases is crucial in many industries, such as mining and petroleum extraction/production. Global demand for H2 continues to increase year after year to meet the needs of these and other industries. The use of H2 has tripled since the 1970s, and the total global market value is expected to exceed $160 B by 2026.
The increased use of H2 also raises the potential for significant events that risk the health and safety of employees and expose businesses to financial liability, especially if leaks go undetected:
- Exposure to even low H2concentrations can result in nausea, headaches, delirium, tremors, convulsions and irritation of the skin and eyes. At high concentrations, an individual can rapidly lose consciousness. The effects of H2on the central nervous system can cause death in a short amount of time.
- H2is flammable and combustible, and it is frequently used in the same vicinity as other flammable materials and gases. It has lower ignition energy than gasoline or natural gas and, once ignited, can spread rapidly or lead to dangerous fires or explosions.
- Stringent safety and environmental standards have been enacted in most areas, including regulations that require gas detection procedures when exposure is possible. Failure to comply with these regulations could result in penalties and fines.
To protect the workplace, workers and the profitability of the business, employers in industries that utilize H2 gas must have reliable and accurate gas detection sensors. Recent innovations have allowed the development of technology that is superior to pellistor/catalytic bead and NDIR sensors.
MEMS-based hydrogen gas sensors. A new sensor that uses a micro-electromechanical system (MEMS) transducer represents an innovative change in gas detection technology. The development of these sensors was driven by the need to detect hazardous gases, particularly H2, more accurately and to mitigate the risks to employees and businesses.
The initial research was conducted through a partnership with the University of Nevada in Reno, the U.S. Department of Defense and the U.S. Defense Advanced Research Projects Agency (DARPA). The research team wanted to take sensors from an atomic-force microscope and use them for sensing molecules. Due to their small size, the sensors were exceptionally sensitive. The first commercial effort to implement this technology was the Small Business Innovation Research (SBIR) project, “Chemical and explosive vapor detection in shipping containers with remotely accessed microcantilever array sensors.” This project’s vision was to create a sensor installed within shipping containers, capable of sniffing out threats such as chemical weapons, explosives or dirty bombs.
Ultimately, the research team realized that these MEMS sensors could deliver better results than both pellistor/catalytic bead and NDIR sensors, without the drawbacks, for sensing multiple gases simultaneously. The MEMS transducer measures changes in the thermal properties of the surrounding air and gases in its proximity, using an embedded heater and thermometer. The MEMS-based H2 sensors display excellent performance in detecting H2 down to concentrations of 0.2 vol%.
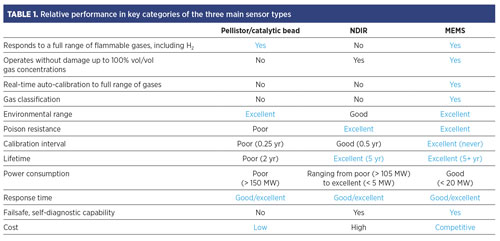
These sensors can measure the concentration of flammable or combustible gases, as well as gas mixtures, and classify the detected gas sample into H2, an H2-containing mixture, methane, light gas, medium gas or heavy gas. The sensors can detect the full range of flammable gases within seconds, from H2 to heavy hydrocarbons. In addition, these sensors have a longer life expectancy (up to 5 yr) and do not need to be recalibrated in the field; a single calibration can deliver an accurate measurement of 15 different gases. With NDIR or pellistor/catalytic bead technology, users would need detectors for each of these 15 gases individually to realize the same results delivered by one MEMS-based sensor. Finally, the MEMS-based sensors are not susceptible to poisoning, even when exposed to high concentrations of gases and a diverse range of environmental conditions. TABLE 1 shows the relative performance of the three main sensor types for key categories.
The MEMS-based gas sensors’ superior performance has been demonstrated in performance tests, along with pellistor/catalytic bead and NDIR sensors. When detecting H2 in a controlled environment, the MEMS-based sensor was able to detect its presence faster and more accurately than the pellistor/catalytic bead sensor. The MEMS-based sensor detected the presence of H2 within 12 sec and accurately reported nearly 50% lower explosive limit (LEL) within 40 sec. The pellistor/catalytic bead sensor did not detect H2 until 30 sec had lapsed, and it never reported an accurate LEL; the highest reading it reported was below 40% LEL. The NDIR sensor did not detect H2 at all. The MEMS-based sensor also outperformed both of the older gas detection technologies when detecting methane and butane (FIG. 2).
MEMS-based gas leak detection sensors are the next generation of combustible gas detection and set a new standard in performance, reliability, accuracy and value. Not only do they represent a tremendous leap forward from NDIR and pellistor/catalytic bead sensing technology, but they also help companies maintain a safe work environment, protect the environment and realize operational efficiencies.
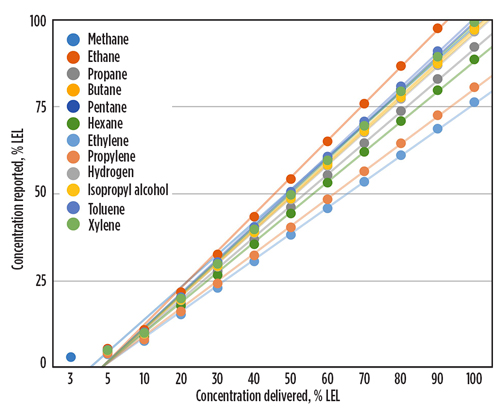
Fig. 2. MEMS-based sensors can deliver accurate, automatic measurements for multiple hazardous gases.
The future of hydrogen and the role of sensors. In the global economy, many countries are focusing on continuous improvement in health, safety and climate-friendly processes and practices. The EU has set a target of 2050 to achieve zero CO2 emissions and reduce emissions by 40% compared to its 1990 baseline. H2 may be the key to achieving these standards, as it can be used as a greener alternative for energy than methane.
Why H2? The present focus is on decarbonizing the gas industry. H2 provides a solution because when it combusts, or burns, the only byproduct it emits is water. For H2 to be a realistic and viable alternative, it must be produced at significant scale and adapted to existing infrastructure. H2 can be produced from methane in large volumes, by two different process methods:
- Steam methane reforming is the most common method for producing bulk H2and accounts for most of the world’s production. This process uses a reformer, which reacts with steam at a high temperature and pressure with methane and a nickel catalyst to form H2and CO.
- Autothermal reforming uses O2 and CO2 or steam to react with methane to form H2. The downside of these two methods is that they produce carbon as a byproduct, so carbon-capture solutions are needed to trap and store this carbon.
A greener alternative is to use electrolysis, which is available on a smaller scale but offers the most significant opportunity. This process uses electricity to split water into H2 and O2. The benefit is that it produces pure H2 with no harmful byproducts.
However, the increased use of H2 must be approached safely. It is a highly flammable gas, and working with it requires that employees and business owners trust the equipment they are using to deliver reliable and accurate results every time. The MEMS-based sensors that are used in molecular property spectrometers represent a leap forward when compared to their gas detection predecessors. Implementing these sensors can ensure that companies maintain the highest possible safety standards and comply with local and national regulations.
LITERATURE CITED
1 International Energy Agency, “The future of hydrogen,” June 2019, online: https://www.iea.org/reports/the-future-of-hydrogen
BEN ROGERS, Director of Engineering, helped launch NevadaNano in 2004, holds multiple patents in sensor technology and is the co-inventor of the Molecular Property Spectrometer. He studied engineering and journalism in college and later earned an MS degree in mechanical engineering. Since that time, he has worked as an analyst at a business accelerator and as a research scientist at various labs, including the University of Nevada at Reno, Oak Ridge National Laboratory and the NASA Jet Propulsion Laboratory. He is the lead author of Nanotechnology: Understanding Small Systems, the first comprehensive college textbook on nanotechnology, and also Nanotechnology: The Whole Story.

