Articles
Hydrogen economy offers low-emissions fuel to combat air pollution
The air quality indexes (AQIs) and particulate matter (PM) concentrations in many fast-developing countries and regions are climbing to high levels that are considered extremely hazardous to public health and the environment. For example, the AQI of New Delhi, India was recently assessed above 300 (severe), and PM2.5 (aerodynamic diameter < 2.5 µm), PM10 (aerodynamic diameter < 10 µm) concentrations also reached extremely hazardous levels.
The reasons for these rising air pollution levels include wind-spread burning of agricultural waste, rising vehicular emissions as car ownership increases; industrial and municipal solid waste emissions; and diesel generator usage, among others. Population explosions in many of these developing regions contribute to pollution increases. Existing climatic and geographical factors may also play a role; for example, in New Delhi (which has a population of around 22 MM people), calm winds, high humidity and a low ventilation index are trapping pollutants in the immediate region.
Several approaches can help overcome these issues:
- A technical decomposition approach for agricultural waste management, using thermal incineration or biological processes to help reduce the quantities of waste and the resulting emissions
- The use of stationary fuel cells, which can provide emissions-free power with no particulate pollutants.
- The use of cleaner fuels and vehicles, such as CNG-fueled and electric vehicles and public transport, as well as hydrogen-fueled vehicles.
This article focuses on the use of hydrogen fuel as a substitute for other fuels to help reduce air pollution in fast-developing regions, such as India.
Benefits of hydrogen fuel. Replacing oil-based vehicle and generator fuels with cleaner fuel sources, such as hydrogen-based fuel, can help cut air pollution drastically. Hydrogen is not only clean-burning and emissions-free, but it also has a much higher energy density (120 MJ/kg) than gasoline (45 MJ/kg).
Hydrogen is a flexible, alternative energy carrier with potential applications across all energy sectors. It can play a significant role in economic and social development in the low-carbon economy.1 At present, worldwide hydrogen production is around 70 MMtpy. Hydrogen is produced from natural gas (48%), crude oil (30%), coal (18%) and water electrolysis (4%).
The hydrogen production process consumes approximately 205 Bm3y of natural gas (6% of global natural gas use) and 107 MMtpy of coal (2% of global coal use), which is responsible for 830 metric MMtpy of CO2 emissions. These emissions can be recouped through the use of hydrogen fuel, which generates very few CO2 emissions. Furthermore, renewable energy sources, such as solar, wind, hydro, nuclear, biomass, geothermal and tidal energy, can be used in the production of commercial-scale hydrogen, making the process even cleaner.
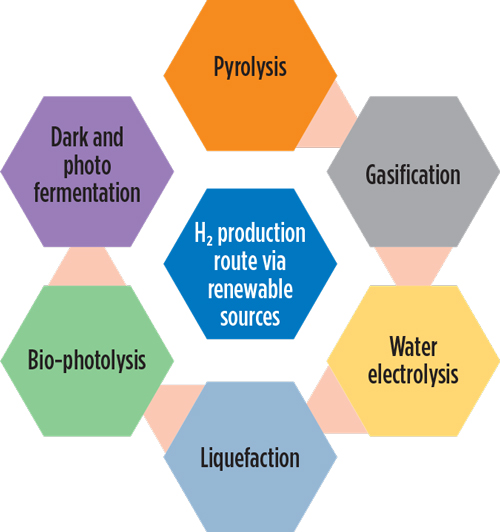
Hydrogen is an energy storage medium, as well as an energy carrier. It has multiple fuel uses—hydrogen vehicles, stationary power sources, building heating, industrial feedstock (e.g., oil refining processes, as well as fertilizer and methanol production) and industrial energy. FIG. 1 depicts the application of hydrogen in various sectors, and FIG. 2 depicts hydrogen production through renewable sources.
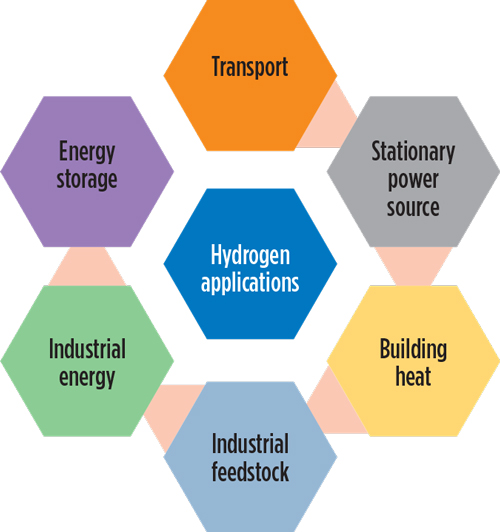
FIG. 1. Applications of hydrogen in various sectors.
|
FIG. 2. Multiple routes exist for hydrogen production. |
Hydrogen production routes. Several technologies have been developed to produce hydrogen from renewable and non-renewable sources, as detailed in the following sections. Many energy stakeholders and producers are focusing on carbon-free energy production to partially replace fossil fuel-based energy production. Renewable technologies include biomass processes (like pyrolysis, gasification, liquefaction, bio-photolysis, dark and photo-fermentation) and the water splitting method.
Steam methane reforming. Among the non-renewable technologies, steam methane reforming (SMR) is the most established process for large-scale production of hydrogen, with conversion efficiency of 74%–85%. It comprises 96% of global hydrogen production and is the cheapest production route. However, SMR uses fossil fuel as feedstock and emits high concentrations of greenhouse gases.
Biomass-to-hydrogen. Biomass comes from agricultural waste, wood-based residues, dedicated energy crops, algae and the organic portion of municipal solid waste and biosolids, such as sludges. Typically, 30 kg–40 kg of biomass is required to produce 1 kg of hydrogen.
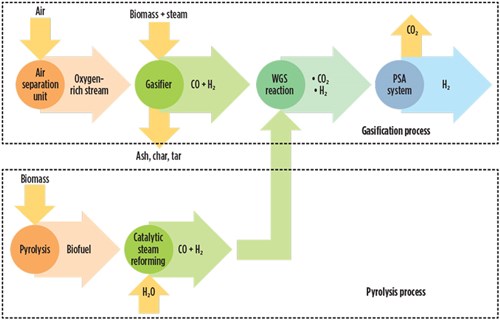
Two types of processes are used for the production of hydrogen: biomass pyrolysis and gasification. The biomass pyrolysis process offers the possibility of obtaining several products, such as bio-oil, biochar, carbon, charcoal and syngas. Biomass can be transformed into hydrogen and hydrogen-rich gases by thermochemical processes. Generally, a thermochemical process is classified as pyrolysis and gasification. Biomass pyrolysis is the thermal decomposition of biomass occurring in the absence of oxygen at a temperature of 350°C–550°C at 0.1 MPa–0.5 MPa to produce biochar, bio-oil and gases including methane, hydrogen, carbon monoxide, and carbon dioxide.2 FIG. 3 shows the biomass pyrolysis process.
|
FIG. 3. Schematic diagram of the biomass gasification and pyrolysis process. |
Basic biomass pyrolysis equations are shown in Eqs. 1 and 2:
CnHm + nH2O (+ heat) → nCO + (n + 0.5m) H2 (1)
CO + H2O (+ heat) → CO2 + H2 (2)
Typical biomass pyrolysis process efficiency is 35%–50%, depending on the type and quality of feedstock used, the type of catalyst, the temperature level, etc. Generally, this process is preferred at a relatively small scale and accepts a wide range of feedstocks.
A few challenges of this process are tar formation, fluctuating hydrogen quantity due to feedstock impurities, and seasonal availability. The hydrogen production cost of biomass pyrolysis ranges from $1.5/kg–$2.2/kg, depending on the facility and biomass type used.2
Gasification of biomass. Biomass gasification is the thermal decomposition of biomass occurring in the presence of oxygen at a temperature range of 200°C–1,200°C at 1 bar–33 bar. Typical biomass gasification efficiency is 52%, but it depends on the type and quality of feedstock, the type of catalyst, the temperature level, etc. The hydrogen production cost of biomass gasification ranges from $1.77/kg–$2.05, depending on the facility and biomass type. However, hydrogen yields from this process fluctuate due to feedstock impurities, seasonal availability and formation of tar.
Adoption of hydrogen fuel. The hydrogen economy faces several challenges for the replacement of fossil fuels. These challenges include hydrogen production from clean sources, hydrogen storage issues, the hydrogen purity requirement for fuel cells, the infrastructure required for hydrogen production and use, and safety and environmental concerns. These barriers to adoption can be overcome by persistent research, industrial interest, fresh investment and government policies promoting the use of hydrogen fuel.
However, hydrogen value chains can be complex and require cross-sectoral investment coordination, which can multiply risk, especially for new network infrastructure. To this end, governments, energy producers and hydrogen promoters must focus on three key activities:
- Increasing the utilization of low-carbon hydrogen in existing industrial use
- Expanding hydrogen into new applications
- Shortening the lifecycle of hydrogen.3
As hydrogen fuel is increasingly adopted by governments, energy producers and the public, it can be applied in a number of key areas, as outlined in the following sections.
Transportation. In developing countries, transport is a critical contributor to air pollution. Road transport plays a vital role in these growing economies, and the contribution of vehicle emissions to their total pollution levels is only expected to increase.
Electric vehicles can help eliminate this problem. The global primary electric vehicle market is broken down into battery electric vehicles and fuel cell electric vehicles. Fuel cell electric vehicles (FCVs) use a fuel cell to power an onboard electric motor. Typically, FCV systems consist of an electric motor, a fuel cell and a hydrogen tank. The hydrogen in the fuel cell reacts with oxygen from the air to produce electricity, which drives the car. When hydrogen is used in an internal combustion engine to create power and water vapor, it is a completely clean technology and does not generate any greenhouse gases or particulates. FCVs have an extended driving range (more than 450 km) and are widely used as heavy-duty vehicles.
The success of hydrogen in the transportation sector will depend on the development and commercialization of competitive FCVs. The challenge is to develop automotive fuel cell systems that have the following characteristics:
- Lightweight and compact (i.e., have high power densities by both mass and volume)
- Tolerant to rapid cycling and on-road vibration
- Durability to 4,000 hr–5,000 hr in automotive drive cycle
- Able to respond rapidly to transient demands for power (e.g. by being hybridized with a battery or ultracapacitor for electrical storage on the vehicle)
- Able to utilize hydrogen fuel of varying purity.
The main challenges to FCV adoption for commercial use include cost competition between hydrogen and renewable fuels, limited infrastructure/refueling stations, and high fuel cell and vehicle costs.
Stationary fuel cells. Stationary cells are poised to replace diesel-fired generators. They are operated by hydrogen or methane feedstock and have many benefits, such as high efficiency, low noise, very low emissions and little space required for installation. Typically, solid oxide fuel cell (SOFC), molten carbonate fuel cell (MCFC), and phosphoric acid fuel cell (PAFC) are preferred for stationary power applications.
Several world regions (primarily Europe, Japan, South Korea and the U.S.) have installed stationary fuel cells with capacities greater than 200 kW, but at widely varying volumes and rates.4 The world’s largest fuel cell power plant, with a capacity of 59 MW, began commercial operation in 2014 at Gyeonggi Green Energy Park in Hwaseong City, South Korea.
The biggest challenges for stationary fuel cells are cost and cell durability. Costs must be reduced by decreasing catalyst loading, efficiency must be increased by reducing fuel crossover and increasing catalyst selectivity, and the size and cost of the hydrogen storage system must be reduced. Total lifecycle efficiency improvements would reduce emissions during operation. Reduction in device size and higher power density are other key parameters.
Furthermore, a lack of codes and standards has been repeatedly identified as a major institutional barrier in deploying hydrogen technologies and developing a hydrogen economy. Over the past 10 yr, the international hydrogen industry has come a long way in identifying needed standards for the commercialization of hydrogen energy systems, and it has participated with standards development organizations to develop standards for hydrogen fuel and infrastructure.
Much of this standards creation is taking place at the International Organization for Standardization (ISO), in ISO Technical Committee 197: Hydrogen Technologies, with input from national organizations. The ISO TC 197, the ISO TC 22/SC 21: Electric Road Vehicles, and the International Electrotechnical Committee (IEC TC 105: Fuel Cells) are involved in fuel cell standards activities.
Takeaway. At present, hydrogen production systems are heavily dependent on fossil fuels. These systems must switch to renewable fuels to make hydrogen a 100% clean fuel source. Increased investments in renewable energy-based resources will improve air quality and energy security. The following key steps are needed to create a viable hydrogen economy:
- Strategy: Governments, stakeholders and manufacturers should have a clear picture of hydrogen supply and demand over the long term so they can plan investments
- Policy: Governments must create attractive policies, along with subsidiaries for the use of hydrogen fuel, that help producers, suppliers, distributors and users
- Infrastructure: Development of infrastructure for the hydrogen economy is needed, including large-scale production, storage, transportation and distribution
- Research and development: More research must be done on the hydrogen economy’s entire supply chain to reduce costs and improve performance, particularly for electrolyzers, fuel cells, membrane and storage systems, etc. GP
LITERATURE CITED
- Sakthivel, S., “Way forward to carbon-free electricity for e-mobility,” Chemical Industry Digest, June 2019.
- Nikolaidis, P. and A. Poullikkas, “A comparative overview of hydrogen production processes,” Renewable and Sustainable Energy Reviews, Vol. 67, 2017.
- Tata Consulting Engineers, “Towards green mobility,” White paper, online: https://www.tce.co.in/white-papers/
- Weidner Ronnefeld, E., Ortiz Cebolla and J. Davies, “Global deployment of large capacity stationary fuel cells,” Publications Office of the European Union, Luxembourg, 2019, online: https://ec.europa.eu/jrc/en/publication/global-deployment-large-capacity-stationary-fuel-cells
S. Sakthivel is a Senior Process Technologist at Tata Consulting Engineers Ltd. in Mumbai, India. He has experience in process engineering; technology analysis, screening and selection; techno-economic analysis; design of laboratory and pilot experimental setups; process hazard analysis; basic, applied and market research; and powder and science technology. He holds a PhD and has published several papers in national and peer-reviewed international journals.

