Articles
Technical and economic pathways for sustainable H2 production
SPECIAL FOCUS: PATHWAYS FOR SUSTAINABLE HYDROGEN
D. B. ENGEL, Nexo Solutions, NEOX Consulting Division, Houston, Texas
In the past few years, increased focus has been devoted to sustainable energy sources and green fuel alternatives due to a series of social, environmental and health-related concerns. This attention seems to be exacerbated during the COVID-19 pandemic and the rethinking of the way in which we live, the impact we make on the ecosystem and the implications these will have to future generations.
In addition, large corporations are witnessing how sustainable green energy companies are gaining in importance and public favor while directly affecting their bottom lines. “Sustainable energy” indicates that the production and use of the energy does not create harmful effects to people and the environment, such as emissions and waste, insensible water use, deforestation or negative impacts on animal life. This sustainable energy would be replenished at an established rate and should, in theory, be able to do so perpetually, with proper maintenance.
While fossil fuels and carbon-based energy and fuel sources still dominate the market today, alternative energy sources have experienced significant growth in recent years. Among these alternative sources of energy is hydrogen gas (H22). This source has the potential to achieve a high level of sustainability because of its generation pathways.
H2 production modes and sustainability
When talking about H2, some basics must be covered in terms of its properties and environmental impact. H2 is the most abundant element in the universe. On earth, its production can follow a number of different avenues that have been coded by “color,” or production mode. The most-often-used modes are gray, blue and green, which are related to the actual H2 production pathway in terms of carbon emissions (sometimes referred to as carbon footprint).
“Gray” H2 production employs fossil fuels that release carbon emissions into the atmosphere primarily as CO2 or greenhouse gas emissions (GHG). Therefore, gray H2 is not an acceptable pathway to sustainability because of the associated CO2 emissions. “Blue” H2 production is similar to gray H2 in terms of production; however, it uses carbon-capture technology to remove and sequester GHG emissions before they can enter the atmosphere.
A common route for CO2 sequestration is reinjection into the ground; however, it can be argued that CO2 rejection might not be a true pathway to sustainability, as CO2 leaks from the ground can often occur. A portion of gaseous CO2 can be safely stored and utilized in other industrial processes, but there is still a long way to go in terms of creating an effective carbon sequestration or immobilization methodology. Color-coding H2 according to its source sometimes can be misinterpreted because of process details and post-production effects. Nonetheless, “green” H2 should be characterized by having zero GHG emissions during its generation.
Green H2—i.e., H2 produced from renewable energy sources not requiring the use of fossil fuels or any other method that creates byproducts that negatively impact the ecosystem—can, in principle, have zero carbon emissions. Green H2 can be a potential sustainable energy source if only the production aspect is considered. However, if the various steps beyond production are evaluated and the full H2 manufacturing chain is considered, a different perspective may arise, posing a fundamental question: Will H2 ever be truly and fully green, with zero emissions of any type? A positive answer seems difficult because green energy sources, such as hydric (water), geothermal, eolic (wind) and photonic (solar) still have carbon footprints and waste generation. For example, the production of photovoltaic (PV) cells for the conversion of light to electricity are associated with some emissions, as are H2 storage and transmission.
A more realistic approach to the ecosystem impact characterization of energies should be based on their sustainability. Numerous color codes for H2 production have been assigned depending on the production mode. This designation is a good initial approach; however, in some cases, it can be confusing or misleading. For example, pink H2, produced using nuclear energy, is not considered a green energy source, nor can it be considered sustainable even though it has low GHG emissions. This is because, at some point, a nuclear facility and its components will need to be decommissioned. The process can be incompatible with, or damaging to, populations and the ecosystem. In addition, the carbon-intensive cement production process for the construction of these large facilities also should be considered as part of the total carbon footprint, in addition to a number of other factors.
As stated previously, many methods exist to produce H2, and several are under development. However, the focus here is on methods for sustainable H2 production. While most of the pathways to green H2 are still in the earlier stages of development, some are much further along and are even in the initial stages of commercialization. At present, however, more than 95% of the world’s H2 is produced using steam reforming of natural gas (gray H2). This process releases considerable emissions of CO2 into the atmosphere. Blue H2, in contrast, uses the same process in combination with carbon capture and sequestration protocols for trapping and disposing of CO2 emissions.
The issue of sustainability is a topic of significant discussion today. However, it has not been addressed thoroughly due to its multifactor outcome affecting the human population, the ecosystem and the capacity of the environment to sustain a given activity or process with minimal or manageable detrimental effects. At some point in the near future, sustainability will need to be precisely defined, with metrics in place to truly assess the sustainability of H2 production.
Electrolytic methods
One method for green H2 production—and probably the most popular at the moment—is water (H2O) electrolysis using renewable energy sources. This method breaks apart (splits) H2O into H2 gas and oxygen gas (O2). On a fundamental level, this process is water-intensive with approximately 2.4 gal of water needed to produce 1.8 lb of H2 gas, assuming minimal losses. In addition, the electrodes for the process use specific metals from mining operations that are not only water-intensive but also carbon-intensive, generating considerable waste. The overall sustainability for this method still requires improvement. FIG. 1 shows an example of a common electrolytic cell used for splitting water into H2 gas and O2 gas.
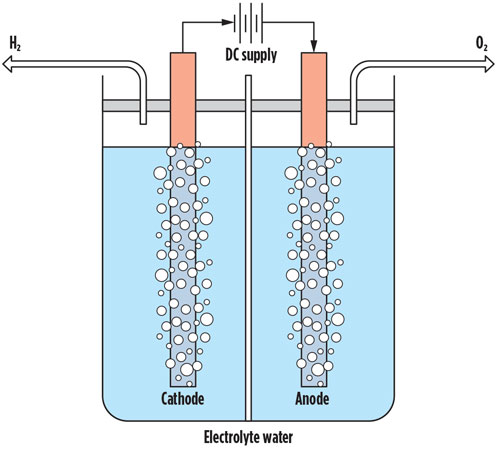
Fig. 1 Example of a common electrolytic cell for water splitting.
Pathways to obtain the most sustainable H2 to date (green H2) via water electrolysis generate the purest H2 at > 99.9% purity. This process alone can be conducted using several different methods and can be carried out at numerous different geographical locations. The most important technologies for water electrolysis are alkaline electrolysis, proton exchange membrane (PEM) electrolysis and solid oxide cell (SOEC) high-temperature electrolysis. FIG. 2 shows a common scheme for a PEM electrolyzer cell. The membrane material is a key feature of PEM cell technology.
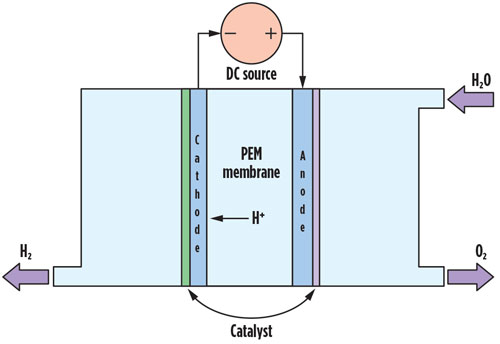
Fig. 2 Diagram of a simplified PEM electrolytic cell.
At present, water electrolysis is the most developed method for green H2 globally and is sold commercially. A number of companies have been installing large-scale electrolyzers in different locations worldwide. The use of renewable energy coupled with water-splitting technology is how this method is considered, to a certain extent, sustainable. Nevertheless, water electrolysis is the shortest-term pathway for achieving highly sustainable H2 production. Corporations and research groups are investigating how PEM can be used more efficiently by lowering the energy requirements for water splitting. This area of research has been more active compared to H2 storage and transportation, which are still carbon-intensive. However, ammonia (NH3) and other alternatives are receiving attention for their potential use as H2 carriers. This is an area of ongoing development that is characterized by a number of challenges, starting with the fact that ammonia has an extremely pungent odor and must be first cracked at the point of use to produce nitrogen and H2.
An alternative method for H2 production is photo-electrochemical (PEC) water splitting. This method uses specialized semiconductors (PEC materials) and light energy to directly dissociate the water molecule into H2 gas and O2 gas. This technology is a long-term pathway due to present technology limitations; however, it holds significant potential for commercial use.
Furthermore, thermochemical water splitting uses high temperature from a concentrated solar power farm to split the water molecule. Water, liquid and vapor are used in this method, in addition to turbines, to create a loop that consumes only water.
Solar and wind pathways
The use of solar energy to produce H2 can be carried out in two main ways:
- Water electrolysis, using solar-generated electricity
- Water splitting with direct solar energy.
When considering solar-generated electricity, PV cells to promote water electrolysis often come to mind. To be practical and for large-scale deployment, the cost of H2 generation via solar energy must be significantly reduced. Previous studies have predicted that achieving a high solar-to-H2 efficiency is a significant driving force for reducing H2 generation costs.
To date, the highest efficiency using a PV water-splitting system is around 12%. Theoretical studies suggest that 25%–30% efficiency can be achieved. Solar-thermal methods via direct dissociation of water employ the high temperatures generated by solar collectors to split water molecules into H2 gas and O2 gas. PEC water splitting is a form of electrolysis, but direct sunlight is used to irradiate a semiconductor immersed in water, which then produces the current used to split water into H2 gas and O2 gas.
Solar energy is not free of challenges. The technology must overcome several hurdles to achieve better sustainability:
- Low light-to-energy conversion efficiency
- Large plot spaces required to assemble solar farms
- Variability of weather conditions
- Fabrication pollution.
Although contamination related to solar energy systems is less compared to other sources of energy, solar energy can produce environmental impacts related to the GHG emissions associated with panel manufacturing, transportation and installation. Additionally, certain hazardous materials and products are used during the manufacturing process of solar PV panels, which can negatively impact the ecosystem.
Wind is an abundant but variable resource for producing electricity. Wind-generated (eolic) electricity also can be used to power water electrolysis systems to produce H2 gas and O2 gas. Wind energy offers a number of advantages over other energy sources; however, it too must overcome several challenges. One advantage is that windpower is often a cost-effective energy pathway. When wind turbines are used onshore as opposed to offshore, they are one of the lowest-priced energy sources available today, with an estimated electricity production cost of $1/kWh–$2/kWh (after tax credits are applied).
Wind is a cleaner fuel source compared to hydrocarbons. It does not contaminate the air compared to power plants that use coal or natural gas, which emit CO2, particulate matter, SOx and NOx. These emissions lead to negative effects such as acid rain, smog and greenhouse effect. Like solar energy, wind energy is considered to be a green and sustainable energy source. In fact, wind energy can be viewed as a variation of solar energy because wind is basically produced by solar heating of the atmosphere.
As long as the sun shines and the wind blows, the energy inherent in them can be harvested. However, windpower and solar energy find challenges to operating under extreme weather conditions. Wind turbines can be noisy and affect the aesthetic of a landscape. Wind farms can also impact local wildlife, such as birds and bats that may fly into moving turbine blades. Additionally, the production of wind turbine blades can be fairly carbon-intensive. The lifespan of these blades is about 15 yr–20 yr. After their lifecycle is complete, some wind turbine blades can be recycled, but others must be landfilled because they are non-recyclable.
Biological pathways
Methods employing biological avenues also have been used to create green and sustainable H2. One method that shows potential is microbial biomass (organic matter) conversion. In this process, microorganisms break down biomass by consuming and digesting its components, releasing H2 gas as a byproduct. This pathway is not in use commercially at present; however, research funding will likely propel the technology over the mid- to long term, and biomass conversion could see large-scale commercialization in the future.
A related method to biomass conversion is photobiological production. This process uses microorganisms in conjunction with sunlight to transform water and organic matter into H2 gas. This pathway is still in the early stages, but it shows promising long-term potential as a highly sustainable H2 production pathway.
Finally, certain types of algae will also produce H2 gas as a byproduct of photosynthesis, requiring only sunlight, carbon dioxide (CO2) and water. Researchers in algal H2 production (sometimes referred to as “olive” H2) are using a series of genetic modification techniques to increase H2 production efficiency in certain algal subsets.
H2 gas also can be produced from municipal solid waste, landfill gas, biogas and waste gas from water treatment plants. These alternative routes do not necessarily lead to green H2 production; however, they add a certain level of sustainability to the entire process of H2 production.
H2 production cost implications
The cost of H2 production in present commercial applications can vary greatly. The lowest H2 costs are associated with non-renewable processes—predominately gray H2 from steam methane reforming (SMR). The cost of H2 production from SMR ranges from approximately $1/lb–$2.5/lb. Future costs using the same method will likely achieve $0.75/lb. If carbon-capture-and-sequestration equipment is in place, $0.11/lb–$0.2/lb should be added to the final cost.
Electrolysis of water in the U.S., using the local electrical grid, would produce H2 at $3/lb–4/lb. Future costs using the same method are estimated at around $1.5/lb–$2/lb. Wind-powered water electrolysis generates H2 at approximately $3/lb–$5/lb. Future costs using the same pathway are estimated at $1.25/lb–$1.65/lb. The cost of solar-produced H2 via electrolysis is presently at $4/lb–$8/lb. Future costs using the same route are estimated at $1/lb–$2/lb.
At present, the cost of H2 production from biomass pathways is approximately $2.5/lb–$3.5/lb; however, large-scale production of H2 using biomass is estimated to cost as little as $0.8/lb–$1.5/lb in the future. H2 production via nuclear thermal conversion of water can achieve a cost of $1.05/lb–$1.5/lb. However, nuclear-powered H2 production technology is not presently considered to be a sustainable or renewable H2 production pathway, and is included here only for comparison purposes.
Takeaway. Sustainable H2 production methods pose many challenges for the future. To start, the term “sustainability” as it pertains to H2 production must be accurately defined, and metrics should be in place to quantify the true sustainability of a given H2 production pathway.
Most H2 production pathways have a carbon footprint and produce an impact to the ecosystem, regardless of their assigned color. Some H2 production pathways are more sustainable compared with others. However, to be able to claim a sustainable (or fully sustainable) H2 production method, the complete production sequence should be evaluated—from the mining and manufacturing of the raw materials for equipment manufacturing, transportation and installation, to the H2 production itself in addition to storage, transportation and point of use.
At present, wind- and solar-powered water electrolysis are the most sustainable H2 pathways, despite the carbon footprints generated by the construction of their facilities. Nonetheless, H2 is an energy source with minimal impact to the ecosystem, and it will only become greener and more sustainable with better technologies, materials and methods.H2T

DAVID ENGEL has more than 20 yr of experience in a variety of areas of chemical engineering and chemistry. He is the inventor in 21 U.S. invention patents and the author/presenter of more than 100 technical papers and conference presentations. Dr. Engel is the Managing Director of Nexo Solutions Companies and heads the Board of Directors for Exion Systems. He holds a BS degree in industrial chemistry and a PhD in organic chemistry.
Fig. 1. Example of a common electrolytic cell for water splitting.
Fig. 2. Diagram of a simplified PEM electrolytic cell.

