Articles
The energy transition for the oil and gas industry
SPECIAL FOCUS: FUTURE OF HYDROGEN ENERGY
M. AL-MAHMOOD, A. AL-QAHTANI and F. ALWARTHAN, Saudi Aramco, Dhahran, Saudi Arabia
The energy transition is a pathway to achieve net-zero by transforming the energy sector into one that is low carbon while maintaining energy sustainability and security—increasing and utilizing the demand in oil and gas throughout the transition while reducing greenhouse gas emissions. The industry is facing challenges to produce energy in an economical and sustainable way as policy makers seek emissions reductions through carbon pricing and trading. According to an International Energy Agency (IEA) report, the transition of oil and gas should consider three main focus levers: rising demand for energy due to a growing global population; affordable and reliable supplies of liquid and gas, since the industry plays a critical role in economic systems; and reducing the energy emissions contribution in line with the decarbonization movement to achieve net-zero emissions.
The authors’ company is not new to the decarbonization industry, as it has built conventional gas plants to treat the gas from the oil wells, resulting in the cleanest gas to replace oil in the power sector. The company has also invested in recovering waste gas to reduce emissions for environmental purposes. The technologies installed to support emissions reduction were mature and feasible both technically and economically. Improving on previous efforts to further the movement toward transition will require governance and policies to control emissions reduction, clean energy product demand, and technologies that are financially mature.
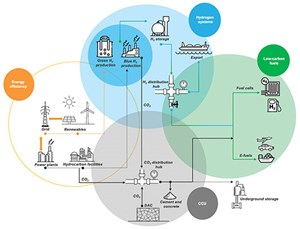
In line with the energy transition movement, the oil and gas industry should consider investment in carbon capture and utilization (CCU), and produce low-carbon products like hydrogen (H2) and ammonia (NH3). Carbon capture will support emissions reduction through flare systems, sulfur oxides (SOx), nitrogen oxide (NOx) and carbon dioxide (CO2) in the boilers, and sulfur recovery unit (SRU) thermal oxidizer stacks. Utilizing emissions to produce low-carbon products will require innovative thinking to support the increasing demand. Carbon capture will also allow the production of blue H2 as a byproduct from steam methane reformers (SMRs) or H2 production technologies. H2 can be utilized either for refueling or to reduce the emissions in the power sector. Analyses show that a beneficial energy transition is more difficult without a supporting government policy shift.
According to the Atlantic Council, recommended steps to support and lead the transition movement in the oil and gas industry include:
- Develop strategies for decarbonization to reduce emissions and ensure profitability
- Support policy development of clear objectives for investors
- Invest in promising projects, technologies, etc., that support achieving net-zero
- Implement approaches to transition oil and gas products to low-carbon products like H2.
A supply chain toward the energy transition has been developed that will focus on four levers, shown in FIG. 1: energy efficiency, a H2 system, carbon capture and low-carbon fuels.
The energy efficiency lever can play a role in reducing emissions and enhancing energy in the power sector, ensuring that operating facilities supply power efficiently to the grid. Renewables are another way to supply clean power to support the grid and achieve the goals of various clean power initiatives. Establishing energy efficiency as a basis will link this lever with the hydrogen system.
Hydrocarbon facilities have a chance to utilize and convert oil and gas sources to H2 (as a clean product) but must capture CO2 sources to achieve blue H2. Renewable power and electrolysis technology will allow the production of green H2 from the demoralized water. The produced H2, whether green or blue, requires storage and transportation routes to supply local demand and exporting purposes.
The captured CO2 sources from hydrocarbon facilities or the air can be collected in a hub to be directly used in the cement and concrete industries (just one potential opportunity to utilize captured CO2) or stored directly underground with specific geological requirements.
Additionally, combining a H2 source (green) with CO2 can be considered under the low-carbon fuel lever umbrella. These sources have the opportunity to produce e-fuel through several licensed technologies. Within the e-fuel section, H2 and CO2 can be converted to methane, diesel, kerosene, gasoline, methanol, DME, etc., as part of synthetic fuel for further utilization. Conversely, H2 can be directly used to produce power through fuel cells technology or as an alternative product for gasoline within the transportation sector. Converting to or moving with this transition requires a set strategy and technology road map with certain criteria. These options allow the oil and gas industry to map its short- and long-term investments.
Achieving the optimum transition in oil and gas will require investment and innovation to reach decarbonization goals and identify clean sources to reduce Scope 1, 2 and 3 emissions.
Pathways to transition. H2—which has been playing (and will continue to play) a major role in global strategies towards decarbonization—can be produced in several ways, depending on the feedstock used. Presently, fossil-based H2 (gray) is the dominant pathway for H2 production using reforming gasification technologies. Several technologies are available to produce H2 from fossil fuels at industrial scale: the three dominant technologies are SMR, autothermal reforming (ATR) and partial oxidation (POx).
SMR is the process of reacting methane (CH4) or natural gas with high-temperature steam as the oxidant in the presence of a catalyst to produce H2, CO and a relatively small volume of CO2. This gaseous mixture is referred to as syngas. The reaction is endothermic and requires heat to the process for the reaction to take place, usually by burning additional natural gas into the reformer furnace.
With POx, CH4 or other hydrocarbons react with a limited amount of oxygen as the oxidant. The oxygen supplied is insufficient to fully oxidize the hydrocarbons to CO2 and water. Since the stoichiometric quantities of oxygen are lower than required, the products of the reaction contain primarily H2 and CO and a relatively small amount of CO2. If air is used as the oxygen source, then nitrogen will also be present in the reaction products—for this reason, the majority of processes use pure O2 from an ASU.
ATR is also a common H2 production technology that combines POx with SMR in a single reaction chamber. The partial oxidation process involves the reaction of oxygen with CH4. The POx of CH4 is a noncatalytic exothermic reaction, while the reforming of CH4 with steam is a catalytic endothermic reaction. The quantity of the oxidant can be adjusted so that the POx reaction provides all the required heat energy for the reforming reaction, eliminating the need for an external input of heat.
When the carbon emissions from the aforementioned processes are captured using CCS technology, then the H2 is termed “blue” to indicate that it is generated by nonrenewable means—the carbon emissions are offset though the use of CCS.
CH4 pyrolysis (turquoise) is another pathway to produce H2 with lower carbon intensity where CH4 is thermally decomposed into H2 and solid carbon. Carbon black is a material produced by the incomplete combustion of hydrocarbons that can be used to form commercial products. The technology has the potential to be completely emissions free (including offsite emissions) if the electrical power is delivered to the process from renewable energy sources.
Clean H2 production (green) can be achieved through the use of electrolysis: electrolyzers use electricity to split water into H2 and oxygen. A typical electrolysis unit consists of a cathode and anode immersed in an electrolyte. When an electrical current is applied, the water is split and H2 is produced at the cathode while oxygen is evolved at the anode. The technology is available commercially but requires further development to reduce the cost of H2 significantly.
CCUS. The authors’ company supports global decarbonization through its own initiatives, including the Corporate Decarbonization Initiative, which aims to reduce the amount of carbon emissions that must be managed to reach a carbon balance or net-zero emissions. One of the key methodologies to reduce carbon emissions are carbon capture, utilization and storage (CCUS) technologies, which capture CO2 emissions at the source or directly from the air. CO2 emissions are then transported away and stored deep underground or turned into useful products. Capturing carbon has been used for decades to help improve the quality of natural gas. Moreover, new ways to add value to waste CO2 are being explored by turning the gas into marketable industrial and commercial products.
Carbon capture technologies can be categorized as:
- Pre‐combustion—Pre‐combustion carbon capture involves the removal of CO2 from fossil fuels before combustion is completed. Examples include coal gasification and SMR, where the feedstock is partially oxidized to form syngas, followed by a water‐gas shift (WGS) to produce a CO2 and H2 stream, from which CO2 can be separated.
- Oxy‐combustion—Oxy‐combustion carbon capture, or oxyfuel combustion, refers to combustion with pure oxygen. In this process, the fossil fuel is burned in oxygen instead of air. The resulting flue gas consists of mainly CO2 and water vapor. The water is condensed through cooling and the result is an almost pure CO2 stream that can be transported and stored.
- Post‐combustion—Post‐combustion capture involves the removal of CO2 from flue gas after the fossil fuel has been burned. Post‐combustion methods are end‐of‐pipe solutions for industrial combustion processes. Flue gases for post‐combustion capture have anywhere from 5%–15% CO2 concentration and are near atmospheric pressure.
CCUS technologies can be classified into three phases in case of deployment:
- Ready technologies are CO2 capture technologies that can be categorized as commercially available or almost commercially available. These technologies have been tested or operated as demonstration projects, or are widely deployed in various commercial applications. In the near or medium term, it is expected that these technologies would involve further development to achieve incremental improvement.
- Emerging technologies, such as emerging CO2 capture technologies, can be demonstrated at pre‐commercial scale and may become commercially available in the coming years.
- Concept technologies are emerging technologies that are considered to be at a low level of maturity with a long lead time to get to market.
Most ready-deployed technologies are based on post‐combustion CO2 capture and are deployed as part of large‐scale CCS projects at existing power generation plants. Deployment of CO2 capture technology has focused on low‐cost process emissions-based opportunities, including industrial applications such as natural gas processing, cement, iron and steel, and chemicals, as well as power generation plants. Carbon capture processes can be classified according to their gas separation/capturing principles, namely chemical absorption, physical absorption, adsorption, calcium and reversible chemical loops, membranes, [direct air capture (DAC)] and cryogenic separation.
CO2 capture activities have mostly focused on power generation plants—mainly coal‐ and gas‐fired power plants—as these comprise the largest stationary source of CO2 emissions. More recently, industrial applications of CO2 capture have begun to gather momentum, mainly in the steel and cement industries, and (to a lesser extent) in the oil refining and chemicals industries.
CO2 storage involves the production and recovery of CO2 from industrial processes and is typically followed by drying and compression. The captured CO2 can be injected into depleted oil and natural gas fields as enhanced oil recovery (EOR) or stored as sequestration in other deep geological formations, such as saline aquifers. Alternatively, CO2 can be used as a chemical feedstock for e-fuel, curing in cement process and algal biofuels production, among a wide range of CO2 utilization options.
Takeaway. Carbon capture from gas streams is not new. CO2 capture technologies based on chemical solvents (amines) were first commercially deployed in the 1930s to separate CO2 and other acid gases from methane in natural gas production. Prior to the early 1970s, all CO2 captured was vented to atmosphere except for a small portion used or sold for other purposes, such as urea production or beverage carbonation.
The main driver of carbon capture is capture costs or capture abatement in $/MMt of CO2. The cost of CO2 capture from low-concentration sources, such as coal-fired power generation, has been reduced by approximately 50% over the past decade and is decreasing for other applications. The cost of CO2 capture can vary by point source and technology. Fuel transformation applications that produce a concentrated CO2 stream and/or that require CO2 to be separated as an inherent part of the process (such as in natural gas processing) have low CO2 capture costs and have been favored for deployment.
Transitioning from oil and gas to clean energy will require a huge investment. Government policies and regulations, in addition to global awareness, will accelerate the transition to net-zero emissions. Investing in CO2 conversion and utilization, especially by integrating it with existing facilities, will contribute significantly to emissions reduction. To increase the clean energy supply and demand, companies must invest in technology innovation and digitalization. This will significantly reduce the cost of green technologies, a major challenge for energy transition.H2T

MOHAMMAD AL-MAHMOOD is a Process Engineer at Saudi Aramco’s Energy Transition Engineering department, within the Hydrogen Systems Engineering Division. He has 7 yr of experience with Saudi Aramco, which includes company operations and project support along with supporting company movement in the Energy Transition initiative. Al-Mahmood has also participated in the commissioning and process stabilization of a gas processing operating facility. He has also supported the company in its decarbonization movement by evaluating flare gas recovery system (FGRS) feasibility, optimum application selectivity and energy consumption. Al-Mahmood earned a BS degree in chemical engineering from Oklahoma State University.

AYIDH AL-QAHTANI is a team-oriented Process Engineer with demonstrated experience in technical services. He is a chemical engineer with 6 yr of experience in engineering services and technical support. During his filed assessment, he worked in gas plant facilities including but not limited to acid gas removal, dehydration and sulfur recovery. He was involved in operations, process and technical support and led gas plants startups from pre-commissioning all the way to gas production of gas sweetening units. Al-Qahtani holds a Fundamental Engineering certificate.

FAWAZ ALWARTHAN is a Process Engineer working in Saudi Aramco’s Energy Transition Engineering department. He has 7 yr of experience working in the hydrogen generation unit (SMR-based) and hydrocracking unit. Alwarthan is a certified Professional Engineer (PE) from NCESS. He holds a BS degree in chemical engineering from KFUPM with highest honors.

