Articles
How high-performance fluid systems help maximize H2 electrolyzer production
Special Focus: Maintenance and Reliability
A. DOMINGUEZ, Swagelok Company, Solon, Ohio
As the world moves toward a low-carbon energy future, the race for viable fossil fuel alternatives continues, with hydrogen (H2) having significant potential to revolutionize energy. Multiple analysts forecast it will continue its exponential growth as countries worldwide discover how well H2 produced through electrolysis works. The questions surrounding its long-term viability focus on how easily it can be produced and how much global capacity will exist.
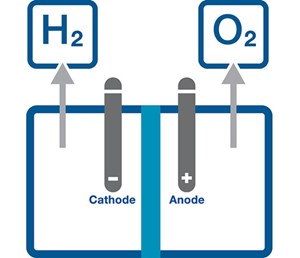
Ensuring reliable H2 production begins with the centerpiece technology of the production process: optimized electrolyzers (FIG. 1). Typically, production facilities can use one of two primary configurations of their electrolyzers [e.g., alkaline and proton exchange membrane (PEM) electrolyzers] to convert water and electricity into H2 and oxygen. Efficient, effective conversion depends on the electrolyzer’s reliable water delivery and functional outflow of the resultant gases.

As engineers design H2 production facilities, they must focus on creating well-constructed fluid systems to ensure peak performance of the plant (FIG. 2). In fact, effective fluid systems can significantly influence the safety, productivity and profitability of H2 production facilities.
Avoiding leaks as the system is installed. The best way to ensure proper fluid system performance is to meticulously design, install and test the systems as the facility is being built. During the final stages of a H2 production facility build, engineers frequently test the crucial fluid systems using factory acceptance testing (FAT) standards. Not only are major components inspected and qualified at the supplier’s site, but the entire system is also often tested by pumping a benign test fluid, such as helium or a mixture of helium and nitrogen, through the tubes at ever-higher pressures to ensure no leaks are present.
Electrolyzer performance is often dependent on leak-free fluid systems keeping the water flowing at adequate levels, and leaks at the end of the process—when H2 and oxygen are captured—may lead to product losses and potentially put employees at risk with an unexpected safety hazard. In addition, other fluids, such as liquid alkaline solutions, may be present in the system, and it is crucial for the reliability of the process and the safety of the operators to have these lines leak free.
If any leaks are detected during FAT:
- The testing process must be shutdown
- The system must be purged of the test gas
- Operators must identify the leak points
- The leaks must be repaired via manual intervention in the system
- The testing must be restarted from the beginning.
Improper installation techniques are the most common reasons leaks exist in fluid systems. Since all leaks must be repaired, they create unnecessary downtime as they are fixed, leading to lower production levels and diminished profitability. Therefore, it is essential to eliminate leaks from the beginning so all new H2 production facilities can succeed.
To prevent leaks during construction, it may make sense to take advantage of any installation training suppliers can provide (FIG. 3). Proper training can help facilities reduce costs during startup. The supplier should also be able to advise on installation best practices so startup delays and expensive redesigning of crucial fluid systems can be avoided. Well-trained installers are less likely to make common mistakes that can lead to a system developing leaks at its connection points.

Keeping H2 output clean. Properly functioning electrolyzers should produce H2 that reaches 99.9% purity if it is to be used as a fuel source for other equipment. Keeping that purity level depends as much on the outflow fluid systems as on the electrolyzer.
The key to maintaining this purity in the post-electrolysis fluid systems is using the highest-quality components possible. The electrolysis process significantly raises temperatures and humidity near the tubing systems, which can damage lesser-quality stainless steel components. In turn, fluid system corrosion can lead to the contamination of the end products (FIG. 4). Damage like corrosion also poses a potential safety hazard because corroded containers are more likely to fail.

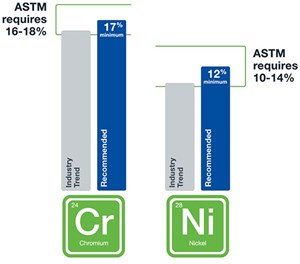
Fortunately, there are several methods operators can use to keep their H2 purity high, including close monitoring of the end products using high-quality gas grab sampling systems or via online monitoring of the quality through an effectively designed system. In addition, the material makeup of the collection containers is critical. To ensure containers are less likely to corrode or suffer from H2 embrittlement, components in these systems should be made of high-quality stainless steels with higher concentrations of chromium and nickel than The American Society for Testing and Materials (ASTM) requires (FIG. 5).
Making sure the facility is as productive as possible. Since the production of H2 as a fuel is growing and evolving, no one knows for certain how to bring a new plant online with guaranteed reliability and cost-effectiveness. Part of what is understood by most facility operators is that adequately executed H2 production requires proper fluid transfer. For this reason, best practices learned from many years of expertise in handling fluid systems can help reduce leaks, safety challenges and maintenance issues in H2 production facilities. Well-managed fuel systems at the ingress and egress of the H2 systems enable electrolyzers to be used to their full capacity. That allows facilities to produce H2 efficiently to meet demand (FIG. 6).

Fewer connection points at the design stage and leak-tightness at the installation phase prevent potentially expensive reconfigurations during FAT and may reduce the number of maintenance issues and related downtime that must be addressed over a system’s lifetime. Reliable inflows and clean outflows allow the process to be as efficient as possible, which can keep the total ownership cost manageable over the production facility's life. Maintaining proper inflows and outflows is another reason operators should pay close attention to the fluid systems supporting the electrolyzer’s function.
The key to operating a successful H2 processing facility is to choose high-quality components and assemblies from the beginning. They should be explicitly designed to handle H2 safely and effectively. Preparing for H2 production from the beginning will enable the facility to get up and running more quickly, safely and productively. In this increasingly competitive green H2 market, speed and precision are critical. A reputable supplier should be able to provide the guidance necessary to ensure the right components are in place to make the operator’s facility a success.
What are the two most prevalent electrolyzer designs? Choosing the right electrolyzer for your facility will depend on which one makes the most sense for your application (FIG. 7).
For H2 production, you have a choice between two main designs:
- Alkaline electrolysis (AEL) electrolyzers. AEL electrolyzers use alkaline electrolysis, a mature technology that has been used for more than 100 yr. Alkaline electrolysis uses a liquid alkaline solution of potassium hydroxide or sodium hydroxide as an electrolyte to conduct electricity, operating at low temperatures. It splits water into H2 and oxygen using this electrical current. It is the cheapest form of H2 production but comes with high maintenance costs.
- PEM electrolyzers. PEM electrolyzers use a solid electrolyte rather than a liquid form to conduct electricity. PEM electrolyzers are more efficient, require less maintenance and take up a smaller footprint than their AEL counterparts. However, PEM electrolyzers are more expensive to operate, as they require precious metals like platinum.
Regardless of which electrolyzer technology is used, the fluid systems that feed and collect the finished H2 are critical to ensuring the electrolyzer always operates at peak performance.H2T
About the author

ANA DOMINGUEZ is the Engineering Services Supervisor - Clean Energy for Swagelok. She has worked for Swagelok for 9 yr and previously held roles as an application and field engineer. Dominguez has been in the clean energy engineering business for more than 13 yr and is familiar with identifying clean energy applications and services, troubleshooting, designing for optimized systems and innovative solutions. She is based in Zurich, Switzerland, and earned an MS degree in chemical engineering and energy management and technology.