Articles
Rethinking hydrogen storage
H2 Storage
M. RUDLOFF, AMBARtec AG, Dresden, Germany
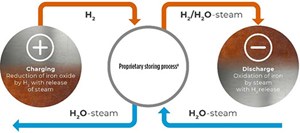
The author's company's hydrogen (H2) storing processa is based on the cyclic reduction of iron oxide as a storage medium by H2 and the oxidation of the iron by water vapor, which again releases H2 from the storage component (FIG. 1). The proprietary storage material consists of a specially designed iron oxide (FeOx) and possesses the unique properties of being reliably and easily reducible and oxidizable, while also exhibiting high thermal stability necessary for long-term usage. The released H2 can then be used directly or converted into electrical energy in a fuel cell, internal combustion engine (ICE) or gas turbine.
During the H2 storage process, H2 can be stored at low- to high-pressure levels (0.5 bar–100 bar) and is fully compatible with any H2 production unit. Fluctuating production rates (depending on wind speed or solar irradiation) are of no concern as the storage processa can adapt readily to changing H2 flows within wide ranges. Additionally, the storage processa is indifferent to residual water contents or gas impurities from H2 production, eliminating the need for any upstream gas treatment stages, particularly alkali metal residues from electrolysis or carbon monoxide (CO)/carbon dioxide (CO2) remaining from gray H2 production, which is critical for other H2 storage technologies (e.g., pressurized or liquified storage).
During the storage process, the H2 reacts with oxygen in an endothermic reduction of the FeOx and is released as water vapor. The endothermic H2 storage reaction requires thermal energy amounting to 3.16 kilowatt hours (kWhr)/kilogram (kg) of H2, which is stored in the storage unit. The storage reaction takes place above 600°C, making it a perfect partner for high-temperature solid oxide electrolyzer cells (SOEC) and raising the efficiency of the SOEC by 10%–15%. If a conventional electrolyzer is used, the water exiting the storage unit is condensed, the energy is extracted and used for H2 heating to reaction temperature, and the water is made available to the electrolysis for renewable H2 production. This leads to significantly reduced operating expenditure (OPEX), especially in arid regions, which are excellent areas for photovoltaic power stations.
The described chemical reaction makes the storage unita unique because the H2 is not stored elementally. Only the potential for facile H2 release is preserved. Consequently, obtaining the required permits and safety licenses can be significantly accelerated. The H2 in the piping and auxiliary equipment during loading and unloading must be considered in any safety review, making the storage solutiona an interesting choice for all safety-sensitive applications (e.g., shipping or on-road mobility). In contrast to cryogenic liquid H2, which exhibits high losses during storage, the author’s company’s technologya provides loss-free H2 storage potential over long periods. Possible benefiting applications are long-range transportation or seasonal H2 storage.
The H2 release can be spatially and temporally separated from the H2 loading process by inserting steam into the storage unit. At > 450°C, the reduced iron reacts with the water molecules, oxidizes to FeOx in an exothermic reaction and simultaneously releases H2. The thermal energy needed during H2 storage is released, amounting to 3.16 kWhr/kg of H2. The remaining steam leaves the storage unit with the H2 in a mixture with more than 90 vol% H2 in the gas stream. The H2 is cooled, the thermal energy is recirculated, and the water vapor is condensed. A H2 quality of 99.9% is achievable using a drying unit with only water as the main impurity. A commercially available water separation system can increase the H2 quality to 99.999% if higher qualities are required. Depending on the intended use of the H2, it can be made available at a pressure of up to 100 bar, independent of the original H2 pressure. For higher pressures (e.g., gas stations), a H2 compressor is required.
Since loading and unloading are high-temperature processes, strict temperature management inside the storage unit is key for successful storage operation. To increase the reaction rate and, thus, the H2 storage and release process speed, a temperature level of > 450°C is targeted. A high-temperature consistency over the entire storage and release process is crucial for continuous process control. Theoretically, there is a risk that the exothermic reaction during H2 release could lead to a local temperature increase at the storage material. This can prevent H2 release or damage the storage material (Eq. 1).
3 Fe + 4 H2O → Fe3O4 + 4 H2 ΔH° = -153 kJ/mol (Eq. 1)
Similarly, during the storage process, a reduction in the temperature of the storage material can be expected due to reaction endothermy, which must be compensated for by external heating or temperature control of the input gas stream (Eq. 2).
Fe3O4 + 4 H2 → 3 Fe + 4 H2O ΔH° = 153 kJ/mol (Eq. 2)
However, the latest experimental results in the pilot facility suggest that the temperature of the H2 storage unit is only slightly influenced by the heat release during the oxidation reaction (H2 release) or the reduction reaction (H2 storage) (FIG. 2).
The multi-zone furnace used was set to constant temperatures for this purpose. FIG. 3 shows the temperature profile in the front, middle and rear reaction zones qualitatively plotted against the progress of the H2 release (left) and H2 storage (right).
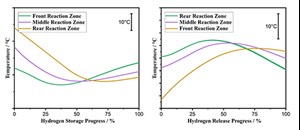
It is clear that with the operation mode applied and near constant heating power of the external heating, there is a high-temperature stability in the storage material. Local hot spots during H2 release or respective cold spots during H2 storage cannot be detected. This means the storage unita can be uniformly loaded or unloaded with H2 without active thermal management, significantly reducing equipment and control requirements.
The author’s company is working on commissioning a scaled-up storagea facility with a storage capacity of 7.5 kg of H2. Delivery of the first storage modules (250 kWhr) is possible in 2023. Storage facilities with a 600 kg of H2/20,000 kWhr capacity will be available from 2024 and planning for individual projects has already begun.H2T
Notes
a HyCS storage process