Articles
Sustainable H2 production through methane pyrolysis
H2 Production Pathways
A. AL-QAHTANI, F. AL-WARTHAN and A. KHANI, Saudi Aramco, Dhahran, Saudi Arabia
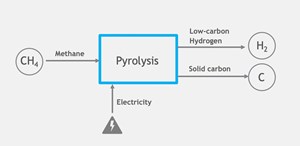
The methane (CH4) pyrolysis process [also known as CH4 cracking or turquoise hydrogen (H2) production] is a thermal (high-temperature) breakdown of CH4 into H2 gas and solid carbon. The process typically takes place above 800°C in the absence of oxygen. The main products of the CH4 pyrolysis process are H2 and solid carbon, commonly known as carbon black, graphite and graphene. The process of CH4 pyrolysis is as follows:
- CH4 feed: CH4 gas is fed into a reactor or furnace and heated to high temperatures without oxygen. This thermal energy breaks the strong bonds between the carbon and H2 atoms in CH4 molecules.
- Pyrolysis reaction: The thermal decomposition of CH4 forms H2 gas and solid carbon. The reaction can be represented as follows (Eq: 1):
CH4 --> C + 2H2 (1)
2CH4 --> C2H2 + 3H2
- H2 production: The H2 produced from CH4 pyrolysis can be used as a clean and versatile energy source. H2 is a valuable fuel that can be utilized in various applications, including fuel cells for electricity generation, industrial processes and transportation.
- Solid carbon generation: The solid carbon (carbon black, graphite and graphene) generated during the CH4 pyrolysis process has several potential uses. It can be utilized as a reinforcing agent in rubber and plastics, as a pigment in inks and coatings, or as a precursor for various carbon-based materials (FIG. 1).
Types of CH4 pyrolysis. CH4 pyrolysis technology is available in three different types in the market: plasma, thermal and catalytic pyrolysis. Plasma pyrolysis is the most mature form and has two methods. The first uses plasma torches to provide heat, and the second uses plasma microwaves to directly ionize the CH4 gas, creating plasma. In thermal (hot) pyrolysis, CH4 is split into H2 and carbon at temperatures above 1,200°C with no catalysts. The main downside of this non-catalytic process is the long cracking times below 1,000°C. In the catalytic pyrolysis process, CH4 breaks down into H2 and carbon over a metal catalyst between 600°C–900°C. Packed bed and fluidized bed reactors are typically considered for catalytic pyrolysis. All pyrolysis types achieve the same objective of producing H2 from CH4 but use different methods.
Carbon products based on the CH4 pyrolysis approach. Various types of carbon material are produced from CH4 pyrolysis, depending on the pyrolysis types and reactor temperature range. CH4 pyrolysis produces solid carbon that can be sold to the market as graphite, graphene and carbon black, offsetting the H2 production cost and preventing the carbon from being emitted as carbon dioxide (CO2). Carbon black and graphite can be used in tires and batteries, while graphene can be used in batteries, electric/photonic circuits, concrete and various medical applications (TABLE 1).
Environmental impact. CH4 pyrolysis offers a potentially significant environmental benefit by utilizing CH4, a potent greenhouse gas (GHG), as a feedstock to produce H2 and solid carbon. The H2 produced via CH4 pyrolysis provides a cleaner alternative to conventional H2 production methods [e.g., steam methane reforming (SMR)], which release CO2 as a byproduct. CH4 pyrolysis enables H2 production without direct CO2 emissions, contributing to decarbonization efforts.
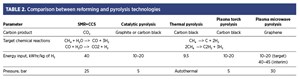
SMR vs. CH4 pyrolysis. The differences between CH4 reforming and CH4 pyrolysis are shown in TABLE 2. Carbon is emitted from an SMR unit as CO2, while in all types of pyrolysis, carbon is captured in a solid form (graphite, graphene and/or carbon black). Pyrolysis eliminates the need for carbon capture and storage (CCS) to produce CO2-free H2. In addition, CH4 pyrolysis requires approximately half the energy required by the SMR process to produce the same amount of H2 with lower H2 pressure output. However, the energy input of SMR and CCS (SMR+CCS) becomes equivalent to the pyrolysis plasma microwave method if the H2 is compressed to 30 bar as an output (TABLE 2). The energy input of SMR+CCS is similar to microwave plasma due to the additional energy costs of the compression. The energy cost ratio to compress CH4 pyrolysis technology is 1 kWhr/kg of H2.
Takeaway. As this field continues to evolve, improvements in efficiency and cost-effectiveness are expected, potentially making CH4 pyrolysis a more viable and competitive option for H2 production in the future, addressing climate change and the transition toward a low-carbon environment. However, it is worth noting that CH4 pyrolysis is an evolving field of research and development, and further advancements are necessary to optimize the process efficiency, scalability and economic viability.H2T