Articles
Impurities in H2: A barrier to clean energy
Special Focus: Fuel Cell Applications
A. RASTOGI, Fluor India, New Delhi, India
Hydrogen (H2)-powered fuel cell electric vehicles (FCEVs) offer enormous potential for clean, carbon-free transportation. While FCEVs are considered one of the most promising eco-friendly alternatives to conventional combustion engines, challenges will always remain (e.g., the risk of contaminants in the H2 available to consumers at refueling stations). Unlike other fuels where contaminants result in the emission of dangerous pollutants to the atmosphere, contaminants in H2 will impact fuel cell efficiency and life. This means increased energy loss and higher fuel cell production, increasing pollution. It is evident that H2 purity is critical for the success of FCEVs and for achieving net zero carbon dioxide (CO2) emissions by 2050. Contaminants in H2 should not exceed the limits specified in International Organization for Standardization (ISO) 14687. This article addresses the impact of impurities on fuel cells and various measures like purification and repeated monitoring adapted to meet the purity requirements prescribed in the ISO standard.
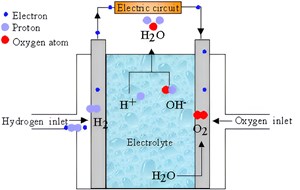
The working principle of fuel cells. Most H2-powered FCEVs use proton exchange membrane (PEM) fuel cells. PEM fuel cells include the electrodes, electrolyte, membrane, catalyst, and gas diffusion layers. H2 fuel is injected into the anode, while oxygen (air) is injected into the cathode side. The catalyst separates the H2 molecule’s electron from the proton. These protons are transferred through the membrane to the cathode, and electrons are directed through the load to produce the electric current (FIG. 1).
Impurities in H2 and the impact on fuel cells. The efficiency and reliability of fuel cells rely primarily on two components: catalysts and PEM electrolysis, both of which are extremely sensitive to the purity of H2 fuel. Various impurities can reduce fuel efficiency, while other impurities can cause severe damage to the fuel cell, shortening its lifecycle. Some examples of these impurities include:
- Carbon monoxide (CO) and CO2 are common contaminants in H2 and can cause impediments in the catalysts, decreasing the fuel cell efficiency, which is difficult to restore.
- Ammonia (NH3) in H2 fuel can affect the membrane conductivity and hinder the proton transfer to the cathode side. This results in poor efficiency and deteriorates the life of fuel cells.
- Sulfur compounds like hydrogen sulfide (H2S) or sulfur dioxide (SO2) are the most harmful contaminants and can cause permanent damage to the fuel cell membrane.
- Water is a relatively common and critical contaminant in H2 fuel. Water itself is not harmful to the fuel cell, but it creates a pathway for other harmful contaminants that have an affinity to water [e.g., NH3 or formaldehyde (CH2O)] to be transported into the H2.
- Other contaminants affecting the fuel cells are oxygen, nitrogen, helium and argon.
Source of H2 impurities. A key source of contaminants in H2 is the H2 generation process itself, where most of the H2 is extracted from natural gas via the steam methane reforming (SMR) process. The SMR process enables the contaminants in the natural gas to remain in the extracted H2, which contains water vapors, sulfur compounds, CO and CO2, NH3 and CH2O. Although these contaminants are monitored and controlled as part of the H2 generation process, they cannot be eliminated from H2.
Other contaminants like nitrogen (N2), O2 (an oxygen molecule with two atoms) and water are added from the atmosphere to the H2 distribution chain through leaks in transfer lines, tanks and valves. H2 produced via water electrolysis is cleaner than that produced by the SMR process, but is still not pure—traces of argon, N2, O2 and H2O remain.
H2 purity standard. Removing the contaminants from H2 to acceptable levels is imperative for the efficient and reliable operation of FCEVs. H2 used as a fuel must meet or exceed the quality requirements of ISO 14687.
Key measures for H2 purity control. Since the key source of contamination is the H2 generation process, suitable purification methods must be used to remove contaminants downstream of the plant. Examples of suitable purification control methods include:
- The most common H2 purification method is pressure swing adsorption (PSA).
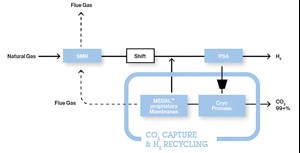
- A proprietary processa that extracts the CO2 from PSA offgases, thereby increasing H2 production (FIG. 2).
- Various detectors and analyzers are used at the originating and delivery points. Gas chromatography and mass spectrometry are the most common methods for analyzing H2 composition and accurately measuring the concentration of impurities.
- H2 purity checks must be required at refueling stations to ensure that the fuel dispensed is safe for use in FCEVs.
- A cavity ring-down spectroscopy analysis detects traces of impurities in H2.1
- H2 produced with electrolysis contains few contaminants compared to the SMR process.
Takeaway. H2 is an important clean energy source that can fuel the global transportation industry, which accounts for about 25% of total delivered energy consumption and 28% of 2021 greenhouse gas emissions.2 However, the acceptability of H2 as a fuel is subject to its purity. If the impurities in H2 fuel exceed allowable limits, transportation costs will increase due to poor efficiency and fuel cell lifecycles will be shortened. Advancement in H2 purification technology and repeated monitoring at each stage of production and distribution will make H2 a reliable fuel.H2T
Notes
a Air Liquide’s Cryocap™ H2
LITERATURE CITED
1 Picarro, “Cavity ring-down spectroscopy (CRDS), online:
https://www.picarro.com/company/technology/crds
2 U.S. Environmental Protection Agency, “Sources of greenhouse gas emissions,” February 2024, online: https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions#:~:text=Transportation%20(28%25%20of%202021%20greenhouse,ships%2C%20trains%2C%20and%20planes
About the author

ANUJ RASTOGI is a mechanical engineer with Fluor Daniel India Private Ltd., New Delhi, and has been with the company for 13 yr. He has more than 18 yr of packaged equipment experience and earned a BS degree in mechanical engineering. The author can be reached at anuj.rastogi@fluor.com.