Articles
From problem to solution: How CO2 enables ultra-low-carbon H2 production
H2 Production Pathways
M. SHELAT, 8 Rivers, Durham, North Carolina (U.S.)
As global industries seek decarbonization solutions, there is an opportunity for hydrogen (H2) to grow from its current niche to provide clean fuel across industries from steel production to long-haul transportation. Realizing this potential will require technologies that produce ultra-low-carbon H2 in an economically viable and commercially scalable way.
Oxy-combustion with inherent carbon capture delivers the economical emissions cuts needed to meet these H2 goals. Reimagining the problem as a solution, the process transforms carbon dioxide (CO2) from a planet-warming waste stream to a working fluid integral to the process, enabling carbon’s inherent capture at a lower cost and lower emissions than comparable technologies.
With > 99% carbon capture at a 10%–15% lower levelized cost of H2 (LCOH) than traditional blue H2 production technologies (such as autothermal reforming with 95% effective back-end carbon capture), oxy-combustion with inherent carbon capture enables the H2 industry to produce ultra-low-carbon H2 in a cost-competitive, commercially scalable way.
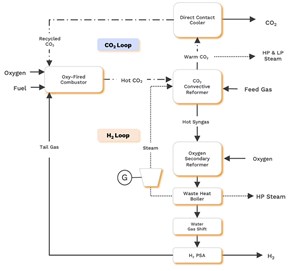
How does oxy-combustion with inherent carbon capture work? The process starts when natural gas and steam convert to syngas in catalyst-filled tubes within a CO2 convective reformer (CCR). High-temperature, high-pressure CO2 provides the reaction’s heat due to the combustion of process tail gas in pure oxygen (O2) with recycled CO2 as diluent, as shown in FIG. 1.
Then, the CCR works synergistically with the oxygen secondary reformer (OSR), which employs autothermal reforming (ATR) to deliver optimal performance. Simply put, by combining the pressurization benefit of ATR and the gas separation benefit of steam methane reforming (SMR), oxy-combustion with inherent carbon capture achieves higher carbon capture rates at lower costs than these conventional technologies alone.
From the OSR, syngas goes to the waste heat boiler, where it cools while generating steam for process use and power generation. Then, the syngas flows through a water gas shift process (CO + H2O → H2 + CO2) for further conversion and heat integration. Next, a pressure swing adsorption (PSA) process separates the H2 from the rest of the cooled tail gas stream (including CO2, some H2, unconverted methane and CO), which is sent to the oxy-combustor.
Tail gas, the byproduct of H2 purification, flows to an oxy-fired combustor where it is burned with an oxidant stream of pure O2 and recycled CO2 to produce a flue gas, principally composed of hot CO2 and water. While remaining isolated from all other process gasses, this flue gas stream flows over the CCR’s catalyst-filled tubes, delivering heat and, therefore, driving the reforming reaction.
In the final step, CO2 exits the CCR at a warm temperature and high pressure. After heat recovery, this stream is sent to a direct contact cooler (DCC) to cool down and remove water, and any CO2 not recycled to the oxy-combustor goes to sequestration. Meanwhile, the H2 PSA separates H2 from syngas and returns tail gas to the combustor.
Stacking up against the competition. Although resembling SMR and ATR processes, oxy-combustion for H2 production is properly understood as its own distinct design paradigm that leverages conventional engineering practices without modifying the fundamental chemical techniques for generating H2 or introducing new infrastructure types—this strategy increases both the technical and commercial viability of new projects. Additionally, unlike these competing technologies, employing oxy-combustion enables the delivery of H2 and pipeline-quality CO2 without the burden of additional back-end capture. Consequently, it affordably achieves lower emissions than conventional blue H2 technologies, while achieving comparable emissions to many green H2 technologies, again, at lower costs (and with far greater scalability).
Both conventional blue H2 technologies, ATR and SMR, struggle to cost-effectively exceed the > 95% carbon capture rate threshold set by RMI (a U.S. think tank formerly known as the Rocky Mountain Institute) for viable new-build technologies designed to mitigate fossil fuel emissions. ATR-based technology relies on back-end capture for carbon capture and cannot cost effectively combust tail gas while capturing carbon emissions, sometimes defaulting to burning H2 product to avoid emissions. SMR-based technology struggles to exceed 90% carbon capture without additional costly post-combustion capture in addition to back-end capture on the syngas production. In contrast, H2 production methods that use oxy-combustion with inherent carbon capture deliver carbon capture rates of > 99% with 5%–7% greater efficiency and 10%–15% lower LCOH.
Conversely, electrolytic H2 offers widely varying carbon intensities at higher costs and lower scalability. Off-grid renewables paired with storage provide the gold standard of carbon intensity, while grid-connected H2 can produce higher emissions than unabated conventional H2 production due to the grid primarily relying on unabated fossil fuels.
The path to scale. According to the International Energy Agency (IEA),1 the world must focus on speed, feasibility and cost-effectiveness to meet its energy transition goals, placing carbon intensity at the forefront.
Innovatively engineered yet scientifically straightforward, oxy-combustion with inherent carbon capture fulfills these parameters by offering a decarbonization solution ready for commercialization today. Thanks to its cost efficiencies, it is affordable without subsidies, freeing decision-makers from concerns that a political swing could jeopardize the viability of a project. Also, by leveraging existing infrastructure and expertise, blue H2 technologies based on oxy-combustion require less “retooling” along the value chain than, say, green H2 technologies, further boosting the feasibility of a pathway that already stands out for its economics.
As is generally understood, co-locating projects with offtakers is the gold standard for minimizing the time, money and emissions spent towards delivering facility exports. Not all offtakers need to be within the H2 industry. For example, the future CO2 sequestration hubs, such as projects within the U.S. Department of Energy’s (DOE’s) Regional Direct Air Capture (DAC) Hub program, offer excellent co-location opportunities, as they will be investing in the infrastructure required for long-term CO2 storage.
Where co-location is infeasible, the next best sites stand near shipping, rail and other transportation networks to transport outputs efficiently to where they are needed.
Industrial decarbonization benefits. Decarbonizing H2 is essential to meet mid-century climate goals and to maximize the industry’s growth potential. McKinsey & Company estimates that clean H2 and ammonia can eliminate 80 gigatons (Gt) of hard-to-abate emissions by 2050, contributing 20% of that year’s total emissions abatement.2
By offering ultra-low-carbon clean fuels at affordable prices, clean H2 via oxy-combustion pathways (and ammonia produced from that clean H2) provides hard-to-abate industries a viable route to decarbonization with or without government incentives. Abating current H2 and ammonia usage across agriculture, energy and industry is the first priority, but clean fuels can also decarbonize industries that are difficult to electrify, such as heavy-duty transportation and high-intensity heat, and industries such as steel and cement production. Plus, ammonia is able to ease H2 transport facilitates transoceanic decarbonization, providing clean fuels to countries that lack the infrastructure or resources needed to fuel their industrial decarbonization. It also enables energy-limited countries to reduce emissions while boosting energy security.
How does this pathway work in practice? Germany, Japan and South Korea offer three examples of countries with highly developed industrial economies but limited space for renewables. Germany plans to import > 70% of its future H2 consumption from overseas strategic partners to decarbonize its industrial economy while boosting its geopolitical resilience.3 South Korea aims to reduce its coal plant emissions through ammonia co-firing in the short term and to replace specific industrial fossil fuel usages entirely with clean fuels in the long term. Japan, another mountainous island nation, is already investigating the conversion of liquified natural gas (LNG) import terminals to ammonia facilities.4
By reimagining the role of CO2, oxy-combustion with inherent carbon capture offers an economical, scalable route to H2 decarbonization that can help the world meet its mid-century emissions targets while providing a path for the H2 industry to achieve its growth potential, independent of government subsidies. H2T
LITERATURE CITED
1 International Energy Agency (IEA), “Global Hydrogen Review 2022,” 2022, online: Global Hydrogen Review 2022 (iea.blob.core.windows.net)
2 McKinsey & Company, Hydrogen Council, “Hydrogen for net-zero,” November 2021, online: Hydrogen for Net Zero | Hydrogen Council
3 Reuters, “Germany's updated hydrogen strategy sees heavy reliance on imported fuel in future,” July 2023, online: Germany's updated hydrogen strategy sees heavy reliance on imported fuel in future | Reuters
4 Atchison, J., Ammonia Energy Association, “Preparing Japan for ammonia imports,” February 2023, online: Preparing Japan for ammonia imports - Ammonia Energy Association
About the author

MAULIK SHELAT is the Senior Vice President, Innovation & Product Development at 8 Rivers. Prior to this position, Dr. Shelat led process gases technology at Air Products for three years with a focus on low-carbon H2 production technology and product development. In the 14 years before his time at Air Products, he worked with Linde developing advanced air separation and oxygen transport membranes for syngas/H2 technology. Prior to his time in the industrial gas industry, Dr. Shelat was an assistant professor and research assistant at universities, as well as spending time with Shaw Group as a heat transfer specialist. He earned his PhD in chemical engineering from the University of Manchester, an MS degree in chemical engineering from The Maharaja Sayajirao University of Baroda, and a BS degree in chemical engineering from Gujarat University. Dr. Shelat holds more than 30 U.S. patents and has been published in dozens of technical publications