Articles
Autothermal and gas-heated reforming for blue H2 production
Special Focus: Advances in H2 Production
M. COUSINS, Johnson Matthey, United Kingdom
As the world intensifies its efforts to combat climate change and reduce greenhouse gas (GHG) emissions, hydrogen (H2) has emerged as a promising cornerstone of the global energy transition. Among the various types of H2 production, blue H2 stands out as a practical and scalable solution to bridge the gap between traditional fossil fuels and a fully renewable future.
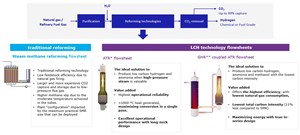
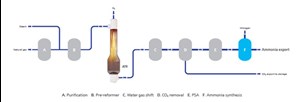
Blue H2 is produced by reforming natural gas with carbon capture and storage (CCS). The author’s company has developed gas reforming technologies, such as an autothermal reformer (ATR)a, and an ATR coupled with a gas heated reformer (GHR)b to produce blue H2. FIG. 1 illustrates an ATR and ATR coupled with a GHR, alongside the traditional steam methane reforming (SMR) option.
ATR flowsheet. The ATR combines two processes that take place in a primary reformer:
- The heating of the process gas (on the air side of the tubes in an SMR)
- The reforming of the feedstock (on the process side of the tube in an SMR).
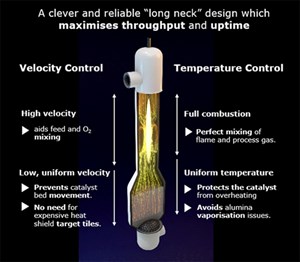
The ATR carries out both functions on the process side of the flowsheet, meaning there is no low-pressure atmospheric carbon dioxide (CO2) release. This is accomplished by introducing oxygen through a burner, which entrains the oxygen flow with the process gas flow. This happens in the area directly below the burner. Simultaneously, the gas stream ignites due to the flammability of the gas mixture and is partially oxidized (burnt), creating heat and resulting in the formation of carbon monoxide (CO) and water (H2O). These processes take place in the neck of the ATR designa, indicated by the flow paths in the ATR neck in FIG. 2.
The hot, well-mixed gas stream then passes through a catalyst bed. It is through this bed the reforming reactions are catalyzed, producing H2 by reacting process gas with steam, as shown by a general reaction in Eq. 1:
CH4 + H2O <=> CO + 3H2 (1)
CO also reacts with H2O in the process to produce H2 and CO2 via the water gas shift process. Due to the elevated temperature (typically 950°C–1,050°C) exiting the ATR, the equilibrium position favors high methane (CH4) conversion, and the kinetics to reach the equilibrium point are elevated. Therefore, when using an active catalyst with properties that protect it from the harsh process conditions, longer life and higher effectiveness can be gained from relatively small volumes of catalyst (VID. 1).
The author’s company’s ATR flowsheeta may be integrated with an ammonia synthesis loop in an analogous way that uses high-grade steam from the secondary reformer. The ATR flowsheet uses the involuntary steam raised from the reform gas boiler(s), exit the ATR, to provide motive steam to power machines in a wider flowsheet.
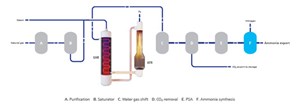
FIG. 3 shows that ATR provides a low level of CH4 slip to exit the ATR and uses pressure swing adsorption (PSA) to remove any trace CO slip from the water-gas shift (WGS) section. The H2 product will have a low inert level. The nitrogen also has high purity because it is a product of the air separation unit (ASU), rather than an air intake that is typically used today.
This means there are less inert gases built up in the loop, lowering the purge and elevating the effectiveness of the loop. This has operational benefits and lowers the capital expenditure (CAPEX) for a given production loop capacity. Further benefits of the ATR flowsheet are:
- It is well-proven in other areas, such as methanol production
- It keeps almost all CO2 at process conditions to enable easy capture for storage
- It produces low-carbon steam that can be used for power
- Its deployment is analogous to the current process, using a similar mix of steam and electrical power
- The benefits from the high-purity gases supplied to the ammonia loop mean loops operate for longer without purge requirement.
The above description of an ATR with a reform gas boilera provides involuntary steam raising. We will now review a flowsheet that can be integrated to provide zero steam export from the H2 production process by use of a GHRb. This enables the increased use of external renewable electrical power. This can lower the carbon intensity of the product and/or lower the cost of the production as less natural gas is needed per unit of ammonia production.
This configuration uses the heat exit in the ATR, to directly drive the reforming reaction in a GHR, where the GHR replaces the reform gas boiler in the ATR flowsheet, using the high-grade heat on the shell side of the GHR to drive approximately 30% of the total hydrocarbon reforming reaction on the tube side of the GHR, before the gas enters the ATR (FIG. 4).
The ATR then completes the remaining 70% of the reforming reaction through the processes described earlier. In this case, the size of the ATR for the same amount of H2 production can be smaller. It follows that the ASU can also be smaller, as less oxygen is required. This has two effects:
- It lowers operational costs (OPEX), as less power is needed for the ASU.
- It is CAPEX neutral, as while the GHR adds a unit operation, the reform gas boiler is removed and the ASU is smaller, resulting in reduced cost.
Some benefits of a GHR/ATR flowsheet include:
- The natural gas requirement per unit of H2 (and ammonia) is reduced by > 10% since no gas is used to raise steam
- The CO2 production is reduced proportionally, lowering transmission and storage costs
- If an operator is aware there will be renewable energy in the future, it allows them to access this benefit months or even years after the flowsheet provides them its first benefits
- The benefits from the high-purity gases supplied to the ammonia loop are still provided.
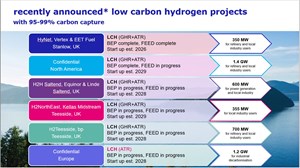
FIG. 5 shows the projects that over the last 2 yr that have selected LCH technology to meet their needs for low-carbon H2 production.
Blue H2 represents a vital stepping stone in the global transition to clean energy. As investments in H2 technologies grow and policy frameworks evolve, blue H2 is positioned to play a crucial role in shaping a more sustainable and resilient energy future, potentially paving the way for green H2. H2T
For more information about Johnson Matthey’s technologies, check out this episode of H2TechTalk.
Notes
a JM’s ATR
b JM’s GHR
About the author
MATT COUSINS is the Licensing Manager for Johnson Matthey, Catalysts Technologies. Johnson Matthey is a specialty chemicals company and a world leader in sustainable process technologies and catalysts. Cousins has more than 20 yr of experience in developing new flowsheets for catalyst manufacturing plants.