Articles
Unlocking low-cost clean H2 for carbon-intensive sectors
Special Focus: Advances in H2 Production
A. GILLIS, Aurora Hydrogen, Alberta, Canada
As some of the world’s most significant emissions contributors, the heavy industry, chemical production, long-distance trucking, off-road vehicles and steel manufacturing sectors can help turn the tide in decarbonizing the economy and mitigating climate change. Still, 84% of industrial companies and 87% of chemical companies are not on track to reach net-zero emissions by 2050.1
Adopting hydrogen (H2) as fuel and feedstock in these energy-intensive sectors is often proposed as a viable solution to reduce their environmental impact. H2 is a zero-emissions fuel when combusted, making it the clear option over traditional combustion fuels with carbon-intensive emissions.
While H2 is a viable energy source, producing the fuel can be an unsustainable process. More than 99.6% of H2 production comes from natural gas reforming and coal gasification, both of which emit high levels of carbon dioxide (CO2).2 To accelerate carbon-intensive sectors’ transition away from conventional fuels and unsustainable H2 production methods, more cost-effective and cleaner forms of H2 production are required. There is clearly significant momentum behind the adoption of H2. Estimates show that subsidies toward low-carbon H2 have surpassed $280 B worldwide in the last 2 yr.3
With various methods of clean H2 production in development, organizations must find the right technology for their business goals and project needs, considering cost, scale, carbon intensity and access to renewable energy, water and CO2 storage. This article examines common methods of clean H2 production, including electrolysis, steam methane and autothermal reforming with carbon capture and storage (CCS) and methane pyrolysis. The methods examined are compared to the author’s company’s microwave-based method of methane pyrolysis that produces clean, distributed H2 at a low cost.
Many clean H2 production methods have emerged to reduce the carbon intensity and create a sustainable fuel source for carbon-intensive sectors. Often referred to as blue, green and turquoise H2, these methods leverage different production pathways to create H2. Each method has its advantages and drawbacks, and it is important for H2 producers to find the technology best fit for their business goals. Here, we explore some of the most common clean H2 production methods and look at the benefits, trade-offs and the project characteristics best suited for the technology.
STEAM METHANE REFORMING (SMR) AND AUTOTHERMAL REFORMING (ATR) WITH CCS
SMR uses high temperatures generated through CH4 combustion to cause a reaction between CH4 and water, creating H2 and carbon monoxide. ATR uses partial oxidation and steam reforming to create synthetic gas (syngas)—carbon monoxide and H2—from natural gas, oxygen and steam. ATR is more energy efficient than SMR, as the process creates its own heat through partial oxidation.
When paired with CCS, reforming processes can prevent 70%─80% of CO2 byproduct.4 While the most recent SMR and CCS plant built in 2020 only has a CO2 capture rate of 29%, other studies have found that more than 90% CO2 capture rates are possible.5,6
Benefits: Integration with existing production infrastructure. Because SMR is already widely used and proven at scale, it is a reliable method to produce H2, but with high-carbon emissions. To produce H2 with a lower carbon intensity, both methods require additional carbon capture infrastructure, along with proximity to CO2 storage.
Trade-offs include:
- Need appropriate geological setting: Carbon capture is only possible in areas with the appropriate geological setting for storage reservoirs. These settings must be porous and permeable, like a deep saline aquifer, and geologically stable for injection into deep storage formations with a cap rock.
- Capital-intensive: SMR and ATR with CCS require CO2 pipelines and proximity to storage. After H2 is produced and CO2 is captured, both products of the process must be transported to the point of use or storage, requiring additional infrastructure and capital expenditure (CAPEX).
- Considerable emissions: About 20%─30% of CO2 emissions go uncaptured from SMR and ATR using CCS but could be reduced with further technological breakthroughs. There is also upstream CH4 leakage that must be factored into the carbon intensity of SMR and ATR.
Project characteristics best fit for SMR and ATR with CCS:
- Existing SMR or ATR Infrastructure: If your project already uses SMR or ATR to create H2, implementing carbon capture could allow for a cost-effective transition to cleaner H2 production, as it does not require entirely new production infrastructure.
- Located near CO2 pipelines or carbon capture sites: SMR or ATR with CCS can be integrated into existing industrial complexes that already require H2 as a feedstock and are located near the appropriate geological setting for storage reservoirs.
- Large scale and high uptime: SMR and ATR produce a steady stream of H2, as they are not fueled with intermittent renewables, making them fit for large-scale projects that require high uptime and a continuous H2 supply.
ELECTROLYSIS
Electrolysis uses electricity to break apart the molecular bonds in water, creating H2 and oxygen gas. This reaction takes place within an electrolyzer, which can come in many different forms and sizes. To produce low-carbon H2, electrolysis must be powered by renewable electricity. It is important to note that non-renewable electricity is currently being used for electrolysis in most projects today, which results in high carbon emissions.
Benefits: Zero direct emissions. When electrolysis uses renewable energy, the only emissions from electrolysis come from the infrastructure required to produce clean electricity, including solar panels, geothermal power plants and wind turbines.
Trade-offs include:
- Energy and OPEX: Electrolysis requires large amounts of renewable electricity to break the H2-oxygen bonds in water, driving up the capital needed to source such large amounts of renewable energy and/or resulting in substantial ongoing operating expenses (OPEX).
- Investment in storage: Because clean electrolysis relies on renewable power, the production method is intermittent, requiring storage for most projects.
- Time to deployment: Electrolysis projects require renewables and grid infrastructure investments, which can take considerable time to plan, permit and construct.
Project characteristics best fit for electrolysis:
- High renewable electricity and water availability: Projects with sufficient amounts of renewable electricity and large water capacity should consider electrolysis as a H2 production method.
- Fit for varying project sizes: While electrolyzers have historically been suitable for small-scale production, recent technological innovation will likely result in larger scale [> 20 megawatts (MW)] deployments over the next several years.
- Intermittent demand: Given the intermittent nature of renewable energy used for electrolysis, projects that do not require a consistent output of H2 could be well-suited for this production method.
METHANE PYROLYSIS
Methane pyrolysis uses heat to break down CH4 into H2 gas and carbon. Unlike SMR or ATR, methane pyrolysis does not produce greenhouse gas (GHG) emissions, instead creating a solid carbon that can be used in various applications. Often referred to as turquoise H2, methane pyrolysis does not require carbon capture and can use renewable energy among other energy types to power the heat source used. The author’s company has created a novel methane pyrolysis methodb using efficient microwave technology to produce clean H2.
Similar to SMR and ATR with CCS, it is important to account for upstream methane leakage when calculating the overall GHG emissions of methane pyrolysis. While the industry is expected to make significant strides on this over the next decade, it is still a factor to consider in a lifecycle assessment.
Benefits: Low, net-zero or net-negative emissions. Because methane pyrolysis produces solid carbon, no CO2 is generated from the process and the H2 can be carbon negative when produced with renewable natural gas and electricity.
Trade-offs include:
- Novel technology: Methane pyrolysis is still in the early stages of development and is not yet proven at scale.
- May require H2 transportation or storage: Methane pyrolysis may require investment in H2 infrastructure depending on the type of electricity used.
- Carbon product: Most methane pyrolysis companies create carbon black as a coproduct. While it can be financially attractive to sell to third-parties, it is a relatively small market that has potential for widespread adoption.
Project characteristics best fit for methane pyrolysis:
- Existing infrastructure for natural gas: Methane pyrolysis primarily uses natural gas as a feedstock, which is widely available. Projects with access to existing natural gas pipelines should consider methane pyrolysis as a clean H2 production method.
- Access to low-carbon intensity electricity: Projects with access to renewable or low-carbon electricity should consider methane pyrolysis as a H2 production method. Because methane pyrolysis is more energy efficient than electrolysis, less renewable electricity is needed.
- Onsite production: Methane pyrolysis typically operates at a smaller scale than SMR or ATR with CCS, making it a great option for projects that have distributed H2 needs.
Producing H2 without CO2 emissions or CO2 pipelines. The author’s company’s methane pyrolysis method produces low-cost clean H2 at the point of use, eliminating the need for costly H2 transportation or CO2 storage. First developed and tested by scientists from the University of Toronto and University of Alberta in Canada, the method uses natural gas and microwaves to produce H2 and solid carbon without CO2 emissions.
There are minimal infrastructure demands for this H2 production method. Because of the highly efficient and distributed nature of the technology, it can leverage existing electricity and natural gas infrastructure to produce H2, dramatically reducing project risks associated with the development of new natural gas, CO2 or H2 pipelines and high-voltage electrical transmission lines. Furthermore, there is no water required, eliminating the environmental impact that SMR, ATR and electrolysis have on local freshwater resources.
Unlike other methods of methane pyrolysis, this method uses direct microwave heating without creating a plasma or requiring a catalyst. This method is extremely efficient, requiring 80% less electricity than water electrolysis, and eliminating the carbon fouling problems that plague many other methane pyrolysis methods while generating a widely applicable carbon product. The components of the technology (fluidized bed reactors and industrial microwave generators) are industrially proven at scale, enabling cost-effective H2 production at low scale while providing a clear scale-up path to meet large industrial H2 demands (FIG. 1).
Chemical reactor houses microwave energy. The methane pyrolysis method occurs in a reactor where carbon particles are fluidized by incoming natural gas. The reactor also acts as a microwave cavity, receiving high-power electromagnetic energy from an efficient industrial microwave generator. Inputs include electricity to power the microwave generator and natural gas, which is mainly comprised of CH4 and similar hydrocarbons, to supply the H2 atoms. No oxygen or water is present in the method, completely eliminating the potential for CO2 generation.
Microwave-heated carbon breaks down CH4. As natural gas enters the reactor, the fluidized carbon particles directly absorb microwave energy and generate enough heat to decompose the CH4 and other hydrocarbons. The methane pyrolysis method is extremely energy efficient due to two properties of microwave energy. First, the conversion of electricity to microwave energy and the absorption of microwave energy by carbon are both highly efficient. This creates an efficient pathway from electricity to heat in the reactora. The reactora exploits the unique heating properties of microwave energy. All reactor materials are either microwave transparent or microwave reflective, which means the carbon particles are the only element in the reactor that absorb microwave energy and generate heat, resulting in precise heating within the reactor.
This combination of heating efficiency and precision results in a H2 production method that requires 80% less electricity per kilogram (kg) of H2 than electrolysis, and significantly less electricity than competing methane pyrolysis methods.
CO2-free H2 and solid carbon. The heating methodb also generates a unique carbon product. Because there is no water or oxygen in the reaction, no CO2 is generated and all carbon atoms in the natural gas turn into solid carbon particles. Most methane pyrolysis methods decompose CH4 directly, creating H2 and carbon dust, commonly known as carbon soot or carbon black. However, the author’s company’s method uses carbon particles as the heat source, CH4 decomposes on the surface of these carbon particles and the carbon particles grow. This has the added benefit of preventing carbon fouling in the reactor, an operational problem that plagues many competing methane pyrolysis methods.
In the reactora, the smaller particles are retained to act as the heat source, and the larger particles are removed on a continuous basis. These sand-sized carbon particles can be utilized in various mass-market applications, such as steelmaking, construction materials, earthworks and as a common feedstock for higher value carbon products like graphene and synthetic graphite. The H2 can be used immediately onsite, eliminating the need for costly H2 storage and transportation, and making it cost competitive with traditional energy sources and chemical fuels.
Efficient electricity consumption. This methane pyrolysis technology uses 80% less electricity per kg of H2 than electrolysis, whether it is wind, solar or other methods of power generation. While this curbs OPEX, the energy efficiency can also have a significant impact on CAPEX and time to market, as it reduces the risk of project delays associated with the construction of electrical infrastructure. In addition, the technology does not require upgrading the grid near a project site, given its limited energy needs. The local grid upgrades not only can delay a project, but they can also represent up to 30% of an electrolysis project’s total CAPEX.
Proven components. The primary technologies used in the author’s company’s production plants—industrial-grade microwaves used in the food processing industry and fluidized bed reactors—are at a technology readiness level (TRL) of 9, meaning they have been tested, proven, sold off the shelf and shipped at scale. For organizations looking to adopt clean H2 and curb emissions immediately, a system that uses proven technology instead of custom parts will increase deployment efficiency, lower risks and prevent supply delays. The solution does not use any precious metals or materials, limiting potential price escalations and supply chain shortages as clean H2 becomes more widely adopted over the next 5 yr.
Scalable units for distributed production. Production plants using this technology are designed to meet industrial demands at the point of use and can be scaled up or down based on project and site requirements. By installing clean H2 production onsite, companies eliminate the need for transportation and storage infrastructure, mitigating significant costs and risks of delay.
No water or wastewater treatment. The microwave technology does not consume water, create wastewater or require water purification. This is particularly useful for projects without access to an inexpensive, purified water source or an inexpensive way of disposing of water. For some projects, water and the associated wastewater can account for > 10% of the total project cost. Water permitting is also a major consideration that can slow down a project considerably.
No H2 pipelines, CO2 storage or CO2 pipelines. The author’s company’s production plants can be constructed at existing natural gas transmission or distribution pipelines and generate H2 at the point of use. They do not require new pipeline infrastructure or H2 transportation, which can increase CAPEX and delay a project’s deployment. Even when a project is located near the geological setting required for CO2 storage, building new CO2 reservoirs and pipelines is expensive and the permitting process takes considerable time. While this option may be attractive for large projects (> 1,000 tpd of H2), medium-sized projects will find the solution more cost competitive.
No small, volatile carbon markets. Due to its larger particle size, the solid carbon that the pyrolysis methodb produces is not restricted to use in niche carbon black markets, such as rubber goods and specialty plastics. This is important because the carbon black market could become saturated with more widespread adoption of methane pyrolysis, ultimately putting projects’ viability and internal rate of return at risk (FIG. 2).
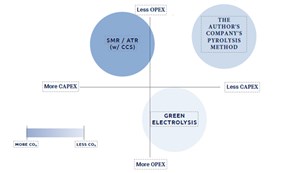
There are many potential pathways to clean H2, each with their own trade-offs. FIG. 3 is an easy-to-visualize, simplified view of the trade-offs between the author’s company’s methane pyrolysis method, electrolysis and SMR or ATR with CCS.
As we have discussed in this article, electrolysis has the potential to have an extremely low carbon intensity—represented by the shade of the circle in FIG. 3. However, the trade-off with electrolysis is the significant OPEX. The high renewable electricity demands for electrolysis can represent well over half of a project's total lifetime costs.
Conversely, SMR or ATR with CCS have the highest carbon intensity of the methods explored in this article. While the OPEX for SMR or ATR with CCS is less than electrolysis, the CAPEX is substantial and the technology should only be considered for the largest projects, and only those with close proximity to CO2 storage.
Takeaway. Over the next decade, the demand for H2 will only accelerate. The International Energy Agency’s (IEA’s) Net Zero Scenario estimates that global H2 demand will increase more than 60% from 2022 to 2030 to surpass 150 MMt, with almost 30% of the demand from new applications.7 However, as long as traditional combustion fuels and conventional H2 production methods remain cheaper than clean H2 production, both H2 demand and decarbonization targets are at risk of falling short. H2T
For more information on Aurora Hydrogen, check out this episode of H2TechTalk: https://h2-tech.com/podcasts/2024/05/producing-h-sub-2-sub-using-natural-gas-and-microwave-energy/
About the author

ANDREW GILLIS is the CEO of Aurora Hydrogen, a Canadian company advancing technology to produce low-cost, low-emissions H2. Gillis has more than a decade of experience in technology development, commercialization, sales and marketing. He has successfully brought several new technologies to market in the resource sector, identifying high-value market segments and building strategic relationships for early adoption. Dr. Gillis earned an MBA from Simon Fraser University and a PhD in engineering from the University of British Columbia.
Notes
a Aurora’s reactor
b Aurora’s heating method
LITERATURE CITED
1 Accenture, “Only a fifth of companies on track for net zero, with heavy industry key to breaking decarbonization stalemate, Accenture reports find,” November 2023. Online: https://newsroom.accenture.com/news/2023/only-a-fifth-of-companies-on-track-for-net-zero-with-heavy-industry-key-to-breaking-decarbonization-stalemate-accenture-reports-find
2 Wood Mackenzie, “The rise of the hydrogen economy,” online: https://www.woodmac.com/market-insights/topics/hydrogen-guide/
3 BloombergNEF, “Hydrogen subsidies skyrocket to $280 billion with U.S. in the lead,” August 2023. Online: https://about.bnef.com/blog/hydrogen-subsidies-skyrocket-to-280-billion-with-us-in-the-lead/#:~:text=Hydrogen%20Subsidies%20Skyrocket%20to%20%24280%20Billion%20With%20US%20in%20the%20Lead,-August%2023%2C%202023&text=Subsidies%20announced%20for%20low%2Dcarbon,the%20latest%20update%20from%20BloombergNEF.
4 Shell, “Quest CO2 capture ratio performance,” February 2020. Online: https://open.alberta.ca/dataset/f74375f3-3c73-4b9c-af2b-ef44e59b7890/resource/c36cf890-3b27-4e7e-b95b-3370cd0d9f7d/download/energy-quest-co2-capture-ratio-performance-2019.pdf
5 Pembina Institute, “Carbon intensity of blue hydrogen production,” August 2021. Online: https://www.pembina.org/reports/carbon-intensity-of-blue-hydrogen-revised.pdf
6 International Journey of Greenhouse Gas Control, “Beyond 90% capture: Possible, but at what cost?,” February 2021.
7 IEA, “Global hydrogen review 2023,” September 2023. Online: https://www.iea.org/reports/global-hydrogen-review-2023