Articles
Safety aspects of H2 installations
Special Focus: H2 Equipment and Services
MP Sukumaran NAIR, Centre for Green Technology & Management, Cochin, India
Hydrogen (H2), particularly green H2, is quickly emerging as a sustainable fuel for mobility and traction for commercial and heavy-duty vehicles like trucks, buses, utility vehicles and trains. Unlike carbonaceous fuels, H2 offers a clean and sustainable alternative, producing only water vapor as a byproduct during combustion or when used in fuel cells. This makes it an excellent option as a transition fuel, and a greenhouse gas (GHG) emissions eliminator that helps combat climate change.
The electrification of heavy-duty vehicles responsible for gross emissions in transportation offers critical challenges related to their size, weight and the long distances they often travel. This requires a higher order of energy density and necessitates frequent charging. The current developments in H2 fuel cells offer longer ranges, lower weight and faster refueling than battery powered electric vehicles. Efficiency and reliability, lower downtime, easier repairs and cost advantages make them suitable for heavy-duty mobility applications in freight transport and public transit.
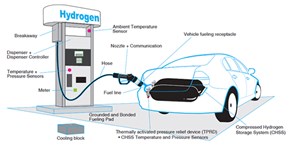
The key challenges in using H2 for mobility and other applications include building the requisite infrastructure comprising generation stations, storages, pipelines, compressors, dispensing stations and fueling outlets, and addressing the associated safety and environmental concerns. An example of H2 storage is shown in FIG. 1. Technologies around H2 production and related infrastructure development are still evolving, and as more countries commit their nationally determined goals toward decarbonization, H2 is poised to play a pivotal role in the future of mobility. This is particularly true in sectors where electrification is difficult and where clean fuels for sustainable mobility pathways are warranted for long-distance, heavy-duty transportation.
Hazards. H2 is highly flammable, has a wide explosive concentration range and is a low-ignition energy—it demands rigorous safety measures during transport and when handling and maintaining H2 vehicles in indoor workshops. A gas leak involving H2 is highly risky. Apart from leaks involving pipeline joints, flanges or ferrules, H2 is also capable of permeation through metals due to its smaller molecular size. Such leaks are difficult to detect and remedy. As soon as H2 reaches the atmosphere and comes into contact with oxygen (O2), it can form explosible mixtures over a wide range of concentrations. As the ignition temperature is low, the mixture can explode even by a mild static spark from the surrounding area.
Workshops, storages and fueling stations are particularly vulnerable to such accidents and must be designed, operated and maintained as per the latest code of practices and by observing relevant safety standards. International Organization for Standardization Technical Report (ISO/TR) 15916:2015 provides the flammability, safety concerns, hazards and risks associated with the handling and storage of H2. It also provides guidelines for safety requirements for the use of gaseous, liquid forms of H2 as well as its storage in either of these forms or as hydrides.
Storage. Physical storage of H2 is in the form of gas or liquid. Commonly employed pressurized tanks are operated around 300 bar–700 bar. The material of construction is usually carbon fiber-reinforced polymer composites, austenitic stainless steels of grades AISI 316 and 304, or steel with aluminum alloy cladding with an overwrap of carbon fiber to impart the necessary strength for the vessel. Austenitic stainless steels AISI 316, AISI 304 and aluminum alloy are usually employed as materials of construction for liquid H2 storage.
H2 can also be stored in solids through chemical and physical bonding. H2 diffuses into metals and is chemically bonded through absorption forming metal hydrides and providing high storage density. Examples include lithium aluminum hydride, sodium boron hydride and certain intermetallic compounds. H2 is also absorbed into the structure of certain metals (occlusion) like platinum and palladium, which are also used as storage for H2. Solid materials like activated carbon, zeolites and certain organo-metallic compounds are also capable of storing H2 in their interstices through physical adsorption, though with a low storge density.
The safety, integrity and reliability of metal and solid storages are critical. Any operational error or failure due to material fatigue could result in a massive release of H2, posing disastrous consequences. Such vulnerabilities are lower in the case of pressurized bullets and cryogenic tank storages. However, transfer points, dispensing stations, compressor stations, valves, flanges and the whole of handling operations are vulnerable to accidents and, therefore, demand risk free operations.
Refueling stations. H2 dispensing stations (FIGS. 2 and 3) are vulnerable to accidents and require extra safety care. Leakage from joints, over-pressurization, and damage to hoses or pipelines are some probable causation events leading to such accidents. A stringent safety protocol comprising specialized procedures for the entire gas handling operation must be in place and periodically checked and assessed for any lapses.
Because H2 is a colorless and odorless gas, major industrial safety equipment manufacturers have developed advanced sensors for H2 leak detection and protection. This preparedness becomes critically important with the heavy-duty transportation of H2.
Hazard area classification. H2 becomes explosive on contact with air or oxygen. The minimum ignition energy for H2-air mixtures is 0.02 mJ (4.8 Kcal) and around 0.0012 mJ (0.29 Kcal) for H2-oxygen mixtures, both of which are extremely low—as stated above, these can be ignited by even small sources of heat or sparks. To guide the selection of appropriate safety equipment, the hazardous area classification for H2 is categorized as Zone 1, Group IIC, T6. Zone 1 indicates the likely occurrence of flammable atmospheres during normal handling operations. Considering its flammability and detonation range, H2 is classified as a Group IIC gas and indicates a high risk of explosion. A T6 rating refers to the maximum surface temperature generated by the equipment, which must not exceed 85°C (185°F).
H2 embrittlement. H2 attack and decarburization are the two types of failures observed in steels used in H2 service. The carbon in the steel reacts with H2 to form methane, resulting in a crack formation called H2 attack. Decarburization is a phenomenon (embrittlement) in which H2 in atomic or molecular form diffuses into the metal lattices, reducing its ductility and causing vessels and pipes to crack and fracture at stresses lower than their yield strength. Both failure modes are favored by high temperature and mitigation lies in selecting appropriate materials and setting optimum operating limits. “Nelson curves” suggest the operating limits for carbon and low-alloy steels in H2 atmospheres at elevated temperature and pressures.
Flammability. When the combustion-related hazards of H2 are considered, three aspects become important: flammability, auto-ignition and detonation. Unlike other combustible gases, the flammability (explosivity) range of H2 in air is wide, with the lower explosive limit at 4 vol% and the higher explosive limit at 77 vol%. These limits are still wider in the oxygen atmosphere, with the higher explosive limit reaching as high as 94 vol%. The detonation limit ranges from 18.3% to 59% at atmospheric pressure. The ignition temperature, also known as auto-ignition temperature of H2 in air—it spontaneously ignites and sustains combustion without any external ignition source like a flame or spark—is 585°C (1,085°F). Detonation limits indicate a narrower range within the flammability range that can initiate and sustain a supersonic combustion wave. This is particularly important when the safety associated with the handling and storage of H2 is considered.
Leak detection. Because it is odorless, H2 cannot be detected by smell. Adding certain odorants that disperse quickly into the atmosphere is a common practice in the case of most other hazardous gases [e.g., natural gas, liquefied petroleum gas (LPG)] to draw attention to the gas leak. No such odorant that is light enough to disperse faster than H2 is available. Therefore, a combination of technologies is often necessary to detect H2 leaks.
The ultrasonic gas leak detection technique allows instant detection of leaks over a large area, making it ideal for outdoor H2 installations. Catalytic point gas detectors, like the one shown in FIG. 4, that can detect H2 concentrations of 0%–10% of the lower explosive limit are available. Advanced sensor technology-based gas detection using electrochemical sensors, radiation frequency flame detectors and infrared sensors provide a high order of selectivity to detect the leakage of H2 even at the ppm level.
Whenever a H2 leak occurs, the gas rises; therefore, gas detectors should be installed at a high level above compressor houses, dispensing stations and roofed storages.
Safe operations. H2 installations are built as per ISO 19880-1, and full hazard and operability studies (HAZOPS) are conducted as per International Electrotechnical Commission (IEC) 61882. All such installations must have robust designs incorporating all relevant codes and American Petroleum Institute (API) recommended practices (RP 686) and built with the right material of construction. Operational risk reduction is determined through a layers of protection analysis (LOPA ) and the required safety integrity levels, ensuring that safety instrumented systems are designed and implemented effectively.
The installation must be fully ventilated, leaving no room for confined spaces and providing an adequate number of leak detection monitors and alarms. Pressure control and relief valves must also be provided. In addition to equipping them with the technical knowledge to operate and maintain the facility, operator training should also instill a strong safety culture in them—this is an important component of the safe operation of H2 installations. Each installation should have a well-defined action plan to mitigate leaks, a readily-available list of emergency procedures and an early warning mechanism to ward off untoward incidents. H2T
About the author
MP Sukumaran Nair is a policy analyst and corporate manager with an extensive technical background and management experience in industry and government. With more than 40 yr of experience, he serves as the Director of the Centre for Green Technology & Management in Cochin. He previously served as Secretary to the Chief Minister, Government of Kerala; Chairperson of the Public Sector Restructuring & Audit Board of the Government of Kerala; and Chairperson and Managing Director of several public sector industries, including fertilizers, chlor-alkali and titanium in Kerala. Dr. Nair’s key specialties include H2 and ammonia. His career has spanned experience as an environment and sustainability researcher, international speaker and contributor to major national and international industry and research journals. He has worked and remains closely associated with professional bodies and serves on several expert advisory committees to the Central and State Governments in India.
The author can be reached at nairmps50@gmail.com.