Articles
Fuel cell technology: A solution for clean power generation
Special Focus: Fuel Cell Applications
M. RENDALL and D. HARVEY, AFC Energy Plc., Cranleigh, Surrey, UK
Fuel cells have often been touted as a clean power technology of the future, offering a solution to the decarbonization challenges for combustion engine vehicles. In the last decade, battery technology has progressed to such an extent that it has become the de-facto first choice for passenger electric vehicles (EVs). However, battery recharging requirements represent a conundrum for heavy fleet vehicles—their weight also results in them being unfeasible for long-distance traveling. With finite energy storage, batteries require frequent recharging, either with a high-powered charger or a long wait on a lower power device. Neither of these are ideal solutions for trucks and vans that must be on the road earning their keep.
Electrification is undoubtedly a key method to decarbonize vehicles; however, the choice of a chemical fuel that combines the benefits of a low-maintenance electric drivetrain and lower emissions is preferable for many users. Fuel cells offer these advantages, as well as high efficiency, rapid refueling and low weight. While this sounds like a perfect solution, the challenge here is that hydrogen (H2) is one of the most difficult fuels to store, transport and handle.
Principles of fuel cell technology. The principles of producing electrical power from fuels dates to Sir William Grove in 1838, when the first uses were to power telegraphs and small watercrafts. While the first fuel cells were quite primitive by today’s standards, they opened a door to the concept of electrochemical power generation that is only limited by the continuous supply of a fuel and an oxidant.
Typically, the preferred fuel chosen is H2, which produces heat energy and water as byproducts when combusted. A fuel cell has the same starting fuel, but instead of making water rapidly (or explosively) by combustion, the process is entirely electrochemical, which is far more efficient but also more complicated to control.
A fuel cell is not dissimilar to a battery, in that the core components consist of an anode, a cathode, a separator (or membrane) that allows only ions to pass through and an external circuit. H2 gas is supplied to the anode, where it undergoes oxidation on the precious metal catalyst, splitting into protons and electrons. The protons travel through the membrane from the anode electrode to the cathode, while the electrons create an electrical current as they move through an external circuit. At the cathode, oxygen molecules react with the incoming protons and electrons to form water. The external electrical circuit allows for current to flow (much in the same way as a battery)—while continuing to provide the electrodes with H2 at the anode and oxygen at the cathode, the current will keep flowing.
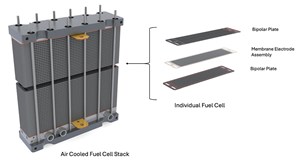
One of the key features of the fuel cell is the membrane. This very thin layer not only keeps the H2 and oxygen separate (preventing a violent interaction), but it also allows protons and water to pass through, making it highly selective. This membrane is often sandwiched and bonded with the anode and cathode electrodes either side, providing good contact but also protecting the membrane from ruptures. The development of this membrane electrode assembly (MEA), shown in FIG. 1, represents one of the most significant improvements over the past few decades, leading to very high current densities (now achieving > 2A/cm2).
However, a persistent, primary challenge with fuel cells is the requirement for H2 without impurities that would block or bind to this selective membrane. Certain chemicals in the fuel or the air flows (such as ammonia or sulfur) can have such a detrimental impact that performance can be significantly degraded with only a few parts per million (ppm).
Fuel cell applications beyond vehicles. Fuel cells can offer significant benefits in applications outside of vehicle propulsion. One area gaining traction is the replacement of dirty off-grid diesel generators with clean fuel cell solutions. Diesel generators often run at very low loads (typically where they are the most inefficient) and require significant regular maintenance—not to mention the carbon emissions pumped into the atmosphere.
Recent work has shown that on a typical construction site, where diesel generators are the most common form of power provision, the reduced maintenance intervals of fuel cells as well as their high efficiency across the load profile can bring the economic cost of running a H2 fuel cell solution close to parity with diesel at today’s current prices of H2. The authors’ company is deploying several fuel cell generators in construction applications that have the same look and feel of a typical generator, but with no noise, smells or emissions. These clean generators will help the construction industry—one of the most polluting in the world—decarbonize and can assist firms win tenders when their carbon footprint is considered during the bidding process.
Challenges and solutions to deploying H2. Unfortunately, the benefits of clean power do not come easily: H2 is a challenging fuel to handle. It is the lightest gaseous element and, therefore, requires an extremely high pressure to store a large, fixed volume. These pressure requirements carry significant cost to compress, store and transport.
Other chemical fuels can be seen as carriers of H2, offering the benefits of low-pressure, high-density fuels that could liberate H2 with simple, straight-forward processes. Two currently favored routes are methanol (CH3OH) and ammonia (NH3). CH3OH contains about 12.6% H2 by weight with NH3 tipping the scales at 17.8% H2 by weight. NH3 also does not contain any carbon atoms in the molecule, meaning that if it is broken apart (cracked) to liberate the H2, the only byproduct is nitrogen (N), which is safely released back into the atmosphere.
NH3 cracking systems, like the one shown in FIG. 2, are currently being developed by the authors’ company’s subsidiarya to enable low-cost H2 to be produced onsite, particularly for construction but also for industrial uses such as furnaces, materials and large-scale power generation.

Will fuel cells ever become mainstream? It is difficult to predict when fuel cells could fully replace conventional power generation solutions. With low-cost H2 now available from NH3 cracking, combined with the significant cost reductions afforded by fuel cell technology, it is the authors’ view that this development might come sooner than anticipated.
The benefits of a clean chemical fuel that emits no carbon dioxide (CO2) and allows for rapid refueling gives rise to a compelling, sustainable, end-to-end clean low-carbon solution. H2T
NOTE
a Hyamtec
About the authors

Dr. Mike Rendall leads the technology development of the NH3 to H2 (NH3 cracking) product lines at AFC Energy. Dr. Rendall has more than 25 yr of experience in leading high-growth technology companies in the H2, electrification and battery sectors.

Dr. David Harvey leads the Fuel Cell Technology and Product Development programs at AFC Energy and is focused on cost reduction and high-volume manufacturing of stacks, systems and generators.