News
Direct power generation from methylcyclohexane using solid oxide fuel cells
Methylcyclohexane (MCH) is very promising as a H2 carrier that can safely and efficiently transport and store H2. However, the dehydrogenation process using catalysts has issues due to its durability and large energy loss. Recently, Japanese researchers have succeeded in using solid oxide fuel cells (SOFCs) to generate electricity directly from methylcyclohexane and recover toluene for reuse. This research is expected to not only reduce energy requirements but also explore new chemical synthesis by fuel cells.
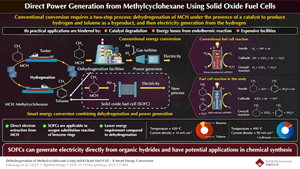
MCH, a type of organic hydride, is expected to be an excellent H2 carrier because it remains liquid at room temperature, is easy to transport, has low toxicity, and has a higher H2 density than high-pressure H2. Dehydrogenation—the process of removing H2 atoms from molecules—in the presence of a catalyst, yields H2 and the byproduct toluene, which can then be used to generate electricity to produce CO2-free power. However, the dehydrogenation reaction is an endothermic reaction, and energy loss as well as the facilities required for the reaction are issues.
Recently, a team of researchers from Japan, led by Professor Akihiko Fukunaga from the Department of Applied Chemistry at Waseda University, has succeeded in generating electricity directly from MCH using SOFC. Their work was made available online on July 4, 2023 in Volume 348 of Applied Energy.
The research team tried to perform two processes simultaneously in a fuel cell: dehydrogenation from organic hydrides, which is an endothermic reaction, and electricity generation, which is an exothermic reaction. To achieve this, they used an anode-supported SOFC with a higher operating temperature than that of a polymer electrolyte fuel cell. They operated it at a temperature that did not allow pyrolysis of organic hydrides and under conditions that prevented carbon deposition at the electrodes.
The production ratio of toluene to benzene was 94:6. This achievement demonstrated the possibility of generating electricity without using dehydrogenation facilities which were conventionally required and using less energy than that required for dehydrogenation reactions using catalysts.
In addition, “It was elucidated that by changing the conditions, oxygen groups could be introduced into the aromatic skeleton using a fuel cell,” revealed Fukunaga.
These results indicate that the MHC reacts with the conducting oxygen ions in the SOFC to successfully generate electricity. Thus, power can be generated directly from MHC, and the energy required for direct power generation is lesser than that required for the conventional catalyst-assisted dehydrogenation reaction of MCH.
“Fuel cells have been studied and developed as devices that produce highly efficient, carbon-free electricity through the electrochemical reaction of H2 and oxygen. In this study, we have demonstrated that this device can be applied to control dehydrogenation reactions from organic hydrides and oxygen substitution reactions of aromatic rings. In the future, new synthetic chemistry may be created by applying fuel cells.” concluded Fukunaga.

