News
Oregon State University research uncovers better way to produce green H2
Researchers at Oregon State University have developed a material that shows a remarkable ability to convert sunlight and water into clean energy.
A collaboration led by Kyriakos Stylianou of the OSU College of Science created a photocatalyst that enables the high-speed, high-efficiency production of H2, used in fuel cells for cars as well as in the manufacture of many chemicals including ammonia, in the refining of metals and in making plastics.
The findings represent a potential new tool to use against greenhouse gas emissions and climate change, said Stylianou, whose research focuses on crystalline, porous materials known as metal organic frameworks, usually abbreviated as MOFs.
Made up of positively charged metal ions surrounded by organic “linker” molecules, MOFs have nanosized pores and tunable structural properties. They can be designed with a variety of components that determine the MOF’s properties.
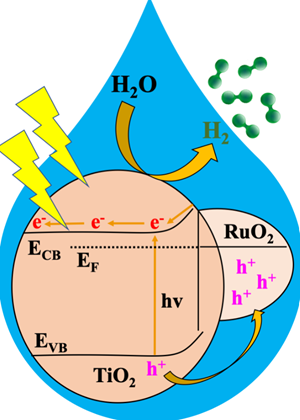
In this study, researchers used a MOF to derive a metal oxide heterojunction – a combination of two materials with complementary properties – to make a catalyst that, when exposed to sunlight, quickly and efficiently splits water into H2.
The heterojunction, which they refer to as RTTA, features MOF-derived ruthenium oxide and titanium oxide doped with sulfur and nitrogen. They tested multiple RTTAs with different amounts of the oxides and found a clear winner.
“Among various RTTA materials, RTTA-1, with the lowest ruthenium oxide content, exhibited the fastest H2 production rate and a high quantum yield,” Stylianou said.
In just one hour, he noted, a gram of RTTA-1 was able to produce over 10,700 micromoles of H2. This process utilized photons—light particles—at an impressive rate of 10%, meaning that for every 100 photons that struck RTTA-1, 10 contributed to H2 production.
“The remarkable activity of RTTA-1 is because of the synergistic effects of the metal oxides’ properties and surface properties from the parent MOF that enhance electron transfer,” Stylianou said. “This study highlights the potential of MOF-derived metal oxide heterojunctions as photocatalysts for practical H2 production, contributing to the development of sustainable and efficient energy solutions.”
Producing H2 by splitting water through a catalytic process is cleaner than the conventional method of deriving H2 from natural gas via a carbon-dioxide-producing process known as methane-steam reforming.
Current catalytic processes for producing H2 from water involve electrocatalysis – running electricity through the catalyst. The sustainability of electrocatalysis depends on using renewable energy, and to be competitive in the market the energy has to be inexpensive.
Presently, methane-steam reforming produces H2 at a cost of about $1.50 per kilogram, compared to about $5 a kilogram for green H2.
“Water is an abundant source of H2, and photocatalysis offers a method to harness the Earth’s abundant solar energy for H2 production,” Stylianou said. “Ruthenium oxide is not cheap but the amount used in our photocatalyst is minimal. For industrial applications, if a catalyst shows good stability and reproducibility, the cost of this small amount of ruthenium oxide becomes less important.”